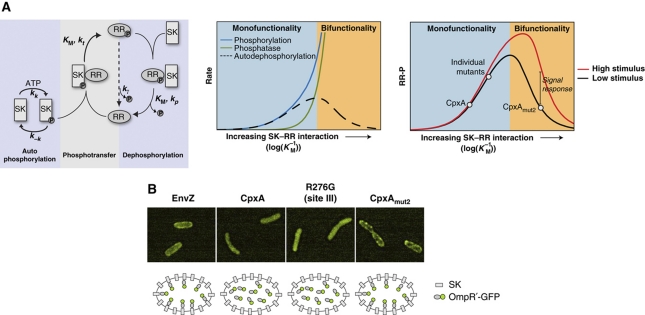
Figure 3.
Emergence of signal response and bifunctionality from increasing SK–RR interaction. (A) Model of two-component signal transduction (see Supplementary information for details). (Left panel) Phosphotransfer and phosphatase rates depend on the strength of the SK–RR interaction, parameterized by Michaelis constants KMt and KMp. For simplicity here we take these constants to be equal (KMt=KMp≡KM). (Middle panel) In the weakly interacting limit, phosphatase activity is negligible (system is monofunctional) and the system is insensitive to stimulus. In the strongly interacting limit, phosphatase activity increases more rapidly than phosphorylation activity. In this limit, the system is bifunctional and responsive to input stimulus. (Right panel) Wild-type CpxA and single-substitution mutants are consistent with weak interactions; CpxAmut2 is consistent with a strong SK–RR interaction. The red curve shows the effects of increased stimulus, which is taken to be a three-fold increase in the autokinase rate kk. The effects of decreasing the phosphatase rate kp are similar (data not shown). (B) Over-expression of EnvZ and CpxAmut2 localize OmpR'-GFP to the cell periphery, consistent with strong interactions between OmpR and these sensor kinases. Deconvolved images are shown. Expression levels of CpxA (WT, single-site mutant, or mut2) were comparable as judged by western blots (data not shown). Cultures of the OmpR'-GFP strain AFS290 (envZ−cpxRA−) containing plasmids that express EnvZ, wild-type CpxA, CpxAR276G, or CpxAmut2 were grown in glycerol minimal medium. See Supplementary information for a detailed description of the strains and growth conditions.