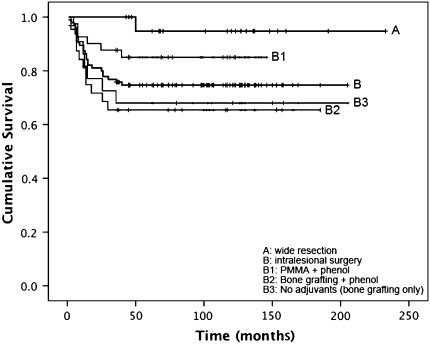
Fig. 2.
Recurrence-free survival for patients with primary giant cell tumor (GCT) treated with wide resection (A) and intralesional surgery (B) is shown. Treatment subgroups for patients were intralesional surgery included the use of polymethylmethacrylate (PMMA) and phenol (B1), the use of bone grafting and phenol (B2), and intralesional surgery without adjuvants (B3). The estimated cumulative recurrence free survival (95% confidence interval) rates were 0.947 (0.847–0.999) for Group A, 0.747 (0.659–0.835) for Group B, 0.851 (0.741–0.961) for Group B1, 0.656 (0.491–0.821) for Group B2, and 0.682 (0.488–0.876) Group B3.