Table 1.
Anticancer activity of retinoid-chalcone derivatives against HT-29 cells.
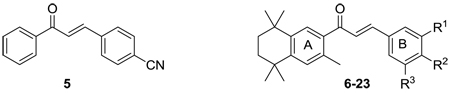 | ||||
|---|---|---|---|---|
| Compound | R1 | R2 | R3 | IC50 ± SD (µM)* |
| 5 | 3.70 ± 0.21 | |||
| 6 | H | CN | H | 1.94 ± 0.57 |
| 7 | H | H | H | 2.86 ± 1.09 |
| 8 | CN | H | H | 0.66 ± 0.25 |
| 9 | H | OCH3 | H | 4.13 ± 1.06 |
| 10 | H | CF3 | H | 2.83 ± 0.35 |
| 11 | H | Br | H | 5.02 ± 0.17 |
| 12 | H | NO2 | H | 1.46 ± 0.22 |
| 13 | H | N(CH3)2 | H | 6.59 ± 0.33 |
| 14 | OH | H | H | 2.66 ± 0.43 |
| 15 | NH2 | H | H | 6.99 ± 1.22 |
| 16 | H | OCH2CHC(CH3)2 | H | 13.33 ± 1.40 |
| 17 | OCH2CHC(CH3)2 | H | H | 3.73 ± 0.07 |
| 18 | OCH3 | H | OCH3 | 1.54 ± 0.16 |
| 19 | OCH3 | OCH3 | H | 8.32 ± 1.59 |
| 20 | NO2 | H | H | 1.45 ± 0.04 |
| 21 | H | 5-1H tetrazole | H | 23.52 ± 1.00 |
| 22 | Cl | Cl | H | 8.12 ± 0.27 |
| 23 | OH | OCH3 | H | 1.59 ± 0.14 |
The experiments were performed twice and the average values were obtained from two independent experiments
