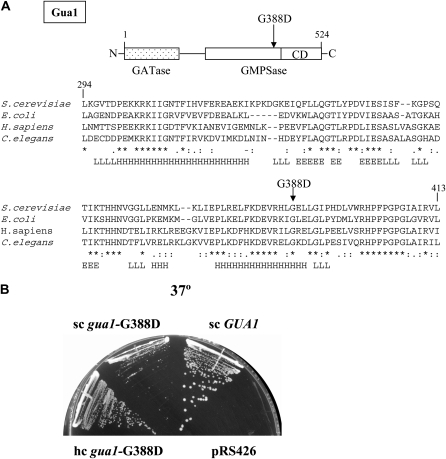
Figure 3.—
Evidence that the gua1–G388D enzyme is partially functional. (A) Schematic representation of the two functional domains of Gua1, glutamine-amido-transferase (GATase) and GMP-synthetase (GMPSase), and location of the G388/D mutation relative to the GMPSase catalytic domain (CD). Alignment of 119 amino acid residues of the Gua1 primary sequence with those of Escherichia coli, Caenorhabditis elegans, and human GMP synthetases, and secondary-structure predictions for Gua1 (H, α-helix; L, loop; E, β-sheet). The G388/D mutation is indicated in boldface type. (B) Transformants of Hm458 (gua1–G388D) carrying empty vector (pRS426), low-copy number plasmids bearing GUA1 (pDI3) and (pDI9), and a high-copy number plasmid bearing (pDI10), were streaked for single colonies on minimally supplemented SD medium and incubated at 37° for 3 days.