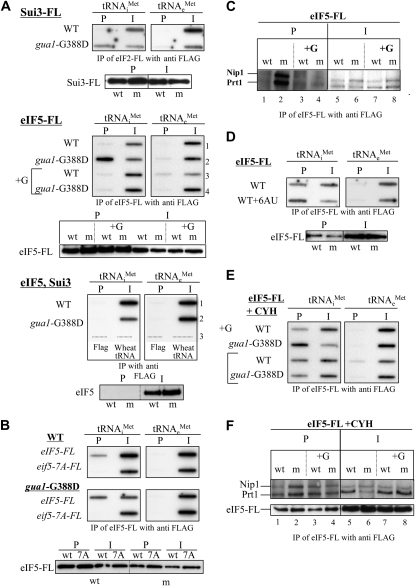
Figure 6.—
Accumulation of initiation factor-tRNAiMet complexes in gua1–G388D cells and in WT cells treated with 6-azauracil (6AU). (A) Methionyl-initiator tRNA (tRNAiMet) associated with flagged-eIF2 (Sui3–FL) and with eIF5–FL accumulates in gua1–G388D cells. WCE were prepared from the following three pairs of isogenic strains: (i) Hm522 (WT) and Hm523 (gua1–G388D), bearing a SUI3–FL-tagged allele, (top); (ii) Hm520 (WT) and Hm521 (gua1–G388D), bearing a TIF5–FL allele (middle); and (iii) H117 (WT) and Hm458 (gua1–G388D), lacking FLAG-tagged genes (bottom). All strains were grown to midlogarithmic phase in liquid SD medium at 28°. Strains Hm520 and Hm521 were also grown in SD containing 130 μm guanine (+G) (middle, lanes 3 and 4). Aliquots of the corresponding WCE were incubated with an anti-FLAG affinity resin for 4 hr at 4°. After intensive washing, RNA from the precipitates (P) and from ∼1% of the immunoprecipitated WCE (I), were extracted and precipitated with ethanol using 50 ng of wheat tRNA as carrier and blotted onto a nylon membrane. Radiolabeled oligonucleotides were used as probes for methionyl-initiator tRNA (tRNAiMet, left) and methionyl-elongator tRNA species (tRNAeMet, right) (materials and methods). The affinity resin and wheat tRNA were independently subjected to the purification process and analyzed with the same probes (bottom, lane 3). Western blot detection of the Flag-tagged proteins showed similar precipitation efficiencies (bottom in SUI3–FL and eIF5–FL sections). (B) An eIF5–7A–FL mutant protein does not co-immunoprecipitate with tRNAiMet. WCE were prepared from transformants of Hm512 (WT) and Hm500 (gua1–G388D) with a low-copy number plasmid containing TIF5–FL (p3147) or the mutant tif5–7A–FL allele (p3148) and co-immunoprecipitation assays conducted as in A. Western blot detection of eIF5–FL and eif5–7A–FL proteins with anti eIF5 antibodies showed similar precipitation efficiencies (bottom). (C) Nip1 and Prt1 subunits of eIF3 co-immunoprecipitate with eIF5 · FL/tRNAiMet complexes in WCE from gua1–G388D cells. WCEs from Hm520 (GUA1, WT) and Hm521 (gua1–G388D, m) were immunoprecipitated as in A. After extensive washing, precipitates (P, lanes 1–4) and ∼5% of the crude extract (I, lanes 5–8) were analyzed by Western using anti-Nip1 and anti-Prt1 antibodies (materials and methods). Growth in minimally supplemented SD containing 130 μm guanine (lanes 3, 4, 7, and 8) is indicated as +G. (D) Accumulation of eIF5 · FL/tRNAiMet complexes in cells treated with 6AU. WCE were obtained from cells of the WT strain Hm520 grown in SD with or without 100 μg/ml of 6-azauracil, and complexes containing eIF5 · FL/tRNAiMet were immunoprecipitated and analyzed by slot blot as above. Western blot detection of eIF5–FL showed similar precipitation efficiencies with and without 6AU (bottom). (E and F) Accumulation of eIF5 · FL/tRNAiMet complexes that contain subunits of eIF3 in WT cells treated with cycloheximide. (E) Detection of tRNAiMet in immunocomplexes with eIF5–FL from WCE of the same strains as in A, middle panels, without and with added guanine (+G), except that 100 μg/ml of cycloheximide (CYH) was added to the cultures prior to the obtention of WCE. (F) The same immunoprecipitates as in E (P, lanes 1–4) and ∼5% of the crude extract (I, lanes 5–8) were analyzed by Western using anti-Nip1, anti-Prt1, and anti-Flag antibodies as in C. Growth in minimally supplemented SD containing 130 μm guanine (lanes 3, 4, 7, and 8) is indicated as +G (top).