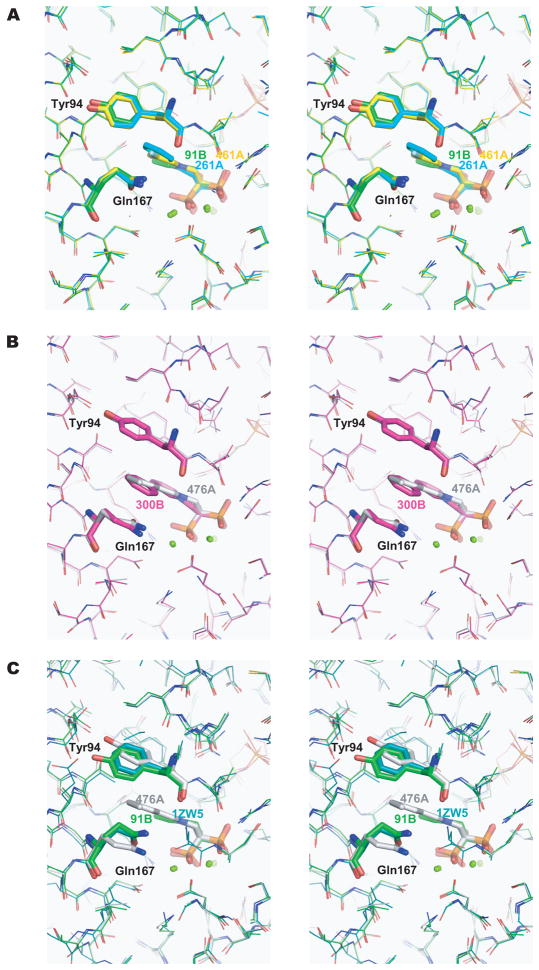
Figure 3. Alternative conformations of Tyr94 and Gln167 (Stereo view).
The conformations of Tyr94 and Gln167 are dependent on the size of the nitrogen-containing group in the bisphosphonate inhibitors. Tyr94, Gln167, and N-BPs are shown as sticks. The same color coding as in Figure 2C is used for different N-BPs. (A) In the structures of TcFPPS in complex with 91B, 261A, and 461A, where the nitrogen-containing groups are shorter, Tyr94 and Gln167 are closer to each other. (B) These two residues become more separated (by ~ 2.0 Å)accommodate the longer nitrogen-containing groups of 300B and 476A. (C) Superposition of the two conformational states of residues Tyr94 and Gln167, as well as the structure of human FPPS/zoledronate (PDB ID: 1ZW5, blue) 14.