Abstract
We conducted a multi-center phase II trial of gemcitabine (G), carboplatin (C), dexamethasone (D), and rituximab (R) in order to examine its safety and efficacy as an outpatient salvage regimen for lymphoma. Fifty-one patients received 2–4 21-day cycles of G (1000mg/m2, days 1 and 8), C (AUC=5, day 1), D (40mg daily days 1–4), and R (375mg/m2, day 8 for CD20 positive disease) and were evaluable for response. Characteristics included: median age 58y (19–79y), stage III/IV 88%, elevated LDH 33%, median prior therapies 2, prior stem cell transplant 12%, chemoresistant 62%, median prior remission duration 2.5 months. The overall and complete response rates were 67% (95% confidence interval [CI], 54–80%) and 31% (95% CI 19–44%), respectively, with activity seen in a broad variety of histologies. Responses occurred in 16 of 17 (94%, 95% CI 83–100%) transplant eligible patients and 15 of 28 (54%, 95% CI 34–71%) with chemoresistant disease. The median CD34 yield in patients attempting peripheral blood stem cell (PBSC) collection following this regimen was 10.9 × 106 CD34+ cells/kg (range, 5.0 – 24.1 × 106). Hematologic toxicity was common but febrile neutropenia (2.5%) and grade 4 non-hematologic adverse events (n=2) were rare with no treatment-related deaths. GCD(R) is a safe and effective outpatient regimen for relapsed lymphoma and successfully mobilizes PBSC.
Introduction
Combination chemotherapy remains the cornerstone of treatment for most newly diagnosed and relapsed/refractory lymphoma patients 1–5 despite the multitude of novel agents that have contributed to improved outcomes in these diseases 6–11. In the relapsed setting, regimens such as ifosphamide, carboplatin and etoposide (ICE), etoposide, methylprednisolone, cytarabine, cisplatin (ESHAP), and dexamethasone, high-dose cytarabine and cisplatin (DHAP) all with or without rituximab (R) are often employed 1–5. Such strategies have yielded response rates of 65–85% and complete response rates of 20–30% in younger, transplant-eligible patients with diffuse large B-cell lymphoma (DLBCL) or Hodgkin Lymphoma (HL). Limitations of these regimens include the inability to safely deliver high-dose cytarabine to older adults, cisplatin nephrotoxicity, ifosphamide neurotoxicity, and the common requirement for aggressive hydration and inpatient hospitalization for delivery of these agents.
Gemcitabine (G), an analogue of cytosine arabinoside, has been shown to have higher affinity for deoxycytidine kinase, increased membrane permeability, longer intracellular tumor retention, and yield higher intracellular concentrations 12. Studies with single-agent G have demonstrated activity in relapsed or refractory lymphoma with response rates ranging from 20 to 70% 13–15. G combination regimens have been explored, but the optimal multi-agent G-based regimen for lymphoma has yet to be defined 16–21.
We hypothesized that based on the known efficacy of G and carboplatin (C) containing regimens for a broad range of lymphomas, the ability to easily administer these agents to outpatients, and the familiarity of community oncologists with this GC regimen in the treatment of solid tumors this study would illustrate a practical, safe and effective treatment strategy for patients with relapsed or refractory lymphoma22–26. Herein we describe the results of a multi-center, prospective phase II trial evaluating the combination of G, C, dexamethasone (D), and rituximab (R), for CD20 + patients [GCD(R)] for patients with previously treated lymphoid malignancies.
Methods
Eligibility
Patients were required to have a relapsed or primary refractory lymphoma with a known World Heath Organization classification 27,28. Eligibility requirements also included measurable disease, a performance status of 0–2, age ≥ 18 years, an absolute neutrophil count (ANC) of ≥ 1,500/μL, a platelet count of ≥ 100,000/μL, a bilirubin and creatinine <1.5 times the upper limit or normal, and a creatinine clearance of > 50ml/min. Study exclusions included known infection with the human immunodeficiency virus, other prior malignancies within 5 years (unless approved by the principal investigator), pregnancy or nursing, inability or unwillingness to utilize contraceptive techniques while on study, active central nervous system lymphoma, or known disease resistance to carboplatin, cisplatin, or gemcitabine. This study was approved by the University of Washington and Fred Hutchinson Cancer Research Center human subjects review boards and all patients provided written informed and verbal consent prior to participation in this study. This trial was registered at clinical trials.gov (NCT00072514).
Treatment Plan
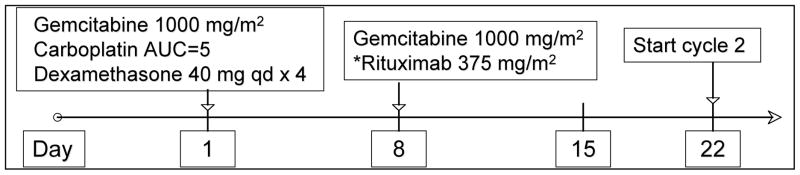
Each 21-day cycle consisted of gemcitabine at 1000mg/m2 on days 1 and 8 (infused intravenously (iv) over 30 minutes), carboplatin at an area under the curve = 5 on day 1 (infused iv over 30 minutes), and dexamethasone 40mg by mouth daily days 1–4. Patients with CD20-expressing lymphoma also received rituximab 375mg/m2 on day 8 (slow iv infusion) as outlined in figure 1. Patients with platelet counts between 50,000–100,000/μL or ANC between 500–1000/μL on day 8 had the G dose reduced by 25% for that cycle only. Patients with platelet count <50,000/μL or neutrophil count < 500 cells/μL on day 8 did not receive gemcitabine. Subsequent cycles could proceed at full dose in patients with an ANC ≥ 1,000 cells/μL and a platelet count ≥ 50,000/μL and a delay for up to 3 weeks for count recovery was allowed. No other dose modifications were allowed. Growth factor support and antibiotic prophylaxis was used at the discretion of the treating physician. A maximum of 4 cycles was allowed on study.
Figure 1. Treatment Schema.
Additional cycles do not begin until ANC is ≥ 1,000/μL and platelet count is ≥ 50,000/μL. Day 8 Gemcitabine given at 75% of full dose if ANC is between 500 – 1,000/μL or platelet count is between 50,000 – 100,000/μL; if ANC is < 500 or platelets are < 50,000, day 8 gemcitabine is not given. Day 8 rituximab* is given regardless of ANC or platelet count. *for CD20 positive lymphomas
Response and toxicity evaluation and definitions
Patients were evaluated by computed tomography at baseline and after the 2nd and 4th chemotherapy cycle. Patients with bone marrow involvement at baseline required reevaluation following therapy to confirm complete remission (CR). Responses were scored according to standard criteria 29. Toxicity was classified and graded according to the National Cancer Institute Common Toxicity Criteria (CTC) Version 2.0. Chemotherapy resistance was defined as not achieving a CR or partial remission (PR) to the last prior regimen. Patients were identified as “transplant eligible” if the treating physician attempted peripheral blood stem cell (PBSC) collection as part of this regimen.
Statistical Design
This study utilized a single-arm phase II design. The pre-specified sample size of 51 evaluable patients provided >90% power to observe a statistically significant difference from the fixed response rate of 30% at the one-sided significance level of .05 if the true response rate was 50% (primary endpoint). Secondary endpoints included overall survival (OS), progression-free survival (PFS), ability to mobilize PBSC, and toxicity. In addition, a stopping rule for safety was employed such that the study would be suspended if sufficient evidence indicated that the true grade 4–5 non-hematologic toxicity rate exceeds 10%. Sufficient evidence was taken to be an observed rate whose lower limit to the associated one-sided 80% confidence interval exceeds 10%. Probabilities of OS and PFS were estimated using the method of Kaplan and Meier 30. Consolidative therapy such as transplant or radiation therapy in the absence of progression was not scored as an event nor were patients censored at the time of consolidation as PFS was not a primary endpoint.
Results
Patients
Fifty-five patients were enrolled into this prospective clinical trial between December 2003 and April 2008 and 51 were evaluable for response (primary endpoint). The 4 non-evaluable patients included 2 with CD20-positive disease who did not receive rituximab as part of their treatment, a third patient who withdrew from the study after 1 cycle of chemotherapy and a fourth patient who was found to be ineligible due to a baseline performance status of 3. The baseline characteristics of the 51 evaluable patients are shown in Table 1. The median age of patients on this study was 58 years (range 19 to 79) with 47% being ≥ 60 years of age. Histologies included 14 with HL, 11 with follicular lymphoma (FL), 8 with DLBCL, 7 with mantle cell lymphoma (MCL), 4 with marginal zone lymphoma (MZL), 3 with peripheral T-cell lymphoma (PTCL), 3 with small lymphocytic lymphoma (SLL), and 1 with lymphoplasmacytic lymphoma (LPL). Patients had received a median of 2 prior therapies (range 1 to 8) and 43 (84%), 7 (14%), and 6 (12%) had relapsed following prior anthracycline therapy, platinum-based therapy, and high-dose therapy and stem cell transplant, respectively. The 6 patients with prior transplant (5 autologous, 1 allogeneic) included 4 with HL, 1 with FL, and 1 with MCL. Eighty-eight percent of patients with CD20-expressing tumors had relapsed following prior rituximab. Twenty-eight of 45 (62%) patients with response data available from their immediately preceding regimen were scored as chemoresistant including 10 (22%) whose disease progressed while receiving their last prior regimen. The median prior remission duration was 2.5 months (range 0 months – 12 years).
Table 1.
Baseline patient characteristics (n=51)
| Median age (range) | 58 (19 – 79) |
| Female | 21 (41.2%) |
| Stage III/IV | 46 (90.2%) |
| Elevated LDH | 17 (34.0%) |
| Median prior therapies (range) | 2 (1 – 8) |
| Prior anthracycline | 43 (84.3%) |
| Prior platinum | 7 (13.7%) |
| Prior rituximab | 29 (88%*) |
| Prior stem cell transplant | 6 (11.8%) |
| Chemoresistant | 28 (62.22%)** |
| Median tumor bulk, cm (range) | 3.7 (1.2 – 13.5) |
percent of CD20+ lymphomas,
based on n=45 with prior response data.
Therapy delivered
One-hundred fifty-nine cycles of GCD(R) were administered with a median of 4 (range 1–4) cycles per patient. Twenty-eight (55%) patients received filgrastim (G-CSF) as primary prophylaxis during cycle 1 and an additional 10 (20%) patients received G-CSF with subsequent cycles as a secondary measure at the direction of the treating MD. Based on the protocol-specific dose adjustment criteria, 130 (82%) cycles proceeded without dose reduction and 131 (82%) cycles were administered without treatment delays. In total 34 of the 51 patients required either dose reduction or delay of at least one cycle. Twenty-five patients were treated at academic sites and 26 at community practices, with no significant differences in protocol adherence.
Response rates, progression-free, and overall survival
Objective remissions were observed in 34 (67%, 95% confidence interval [CI] 54–80%)) patients, including CR in 16 (31%, %, 95% CI 19–44%). Exploratory subset analyses indicated that responses were seen in 12 of 14 (86%, 95% CI 67–100%) HL and 9 of 11 (82%, 95% CI 59–100%) FL, the most frequently treated histologies in this trial. The full details of the responses by histology are described in Table 2. Sixteen of 17 (94%, 95% CI 83–100%) of the autologous transplant-eligible patients responded (8 CR/CRu, 8 PR), as compared to 18 (8 CR/CRu, 10 PR) of 34 (53%, 95% CI 36–70%) of those who were not prospectively thought to be appropriate for transplant. Furthermore, 15 of 28 (54%, 95% CI 34–71%) with chemoresistant disease responded. The details of univariate factors associated with response are shown in Table 3, noting the small sample size in many of these subsets.
Table 2.
Response rates by histology to Gemcitabine, Carboplatin, Dexamethasone, and Rituximab
| Histology | n | Overall response (%, 95% CI) | CR/CRu (%, 95% CI) |
|---|---|---|---|
| HL | 14 | 12 (86, 67–100) | 7(50, 24–76) |
| FL | 11 | 9 (82, 59–100) | 4 (36, 8–65) |
| DLBCL | 8 | 5 (63, 29–96) | 1 (13, 0–35) |
| MCL | 7 | 5 (71, 38–100) | 3 (43, 6–80) |
| MZL | 4 | 3 (75, 33–100) | 1 (25, 0–67) |
| PTCL | 3 | 0 (0, 0–71)* | 0 (0, 0–71)* |
| SLL | 3 | 0 (0, 0–71)* | 0 (0, 0–71)* |
| LPL | 1 | 0 (0, 0–100)* | 0 (0, 0–100)* |
| Total | 51 | 34 (67, 54–80) | 16 (31) |
HL= Hodgkin lymphoma, FL= follicular lymphoma, DLBCL= diffuse large B-cell lymphoma, MCL= mantle cell lymphoma. MZL= marginal zone lymphoma, PTCL= peripheral T-cell lymphoma, SLL= small lymphocytic lymphoma, LPL= lymphoplasmacytic lymphoma.
CI’s are exact confidence intervals
Table 3.
Univariate analysis of factors associated with response to Gemcitabine, Carboplatin, Dexamethasone, and Rituximab
| Factor | Response rate (%) | Odds Ratio for response (95% CI) | p - value |
|---|---|---|---|
| Chemoresistant* | |||
| Yes | 54 | 0.25 (0.06–1.05) | .06 |
| No | 82 | ||
| > 2 prior regimens | |||
| Yes | 73 | 1.63 (0.49–5.42) | .43 |
| No | 62 | ||
| Prior anthracycline | |||
| Yes | 67 | 1.24 (.26–9.96) | .79 |
| No | 63 | ||
| Prior platinum | |||
| Yes | 71 | 1.29 (0.22–7.47) | .77 |
| No | 66 | ||
| Prior rituximab** | |||
| Yes | 62 | 0 (---) | .13 |
| No | 100 | ||
| Prior transplant | |||
| Yes | 83 | 2.76 (.30–25.71) | .37 |
| No | 64 | ||
based on 45 patients with prior response data.
based on 34 with CD20+ lymphoma only
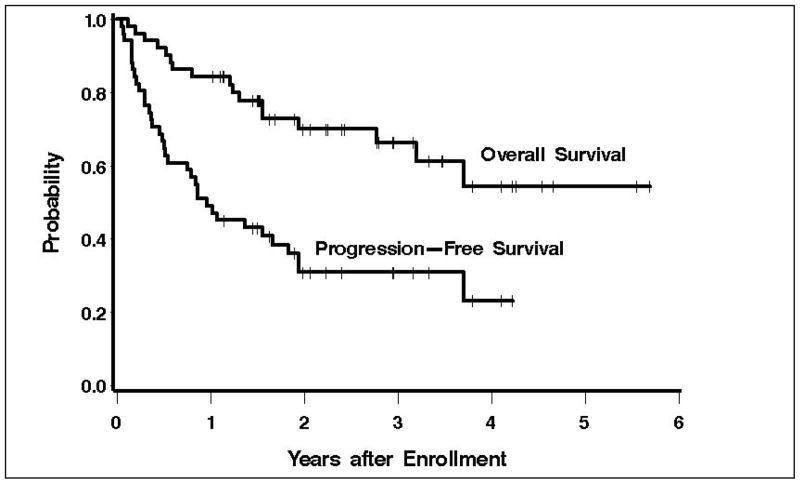
With a median follow-up of 2.4 years, 34 patients are alive, 16 of whom are progression free (Figure 2). The estimated 3-year OS and PFS are 66% (95% CI 50–79%) and 31% (95% CI 18–44%). Ten patients proceeded to autologous transplantation and 4 proceeded to non-ablative allogeneic transplantation following this regimen. Causes of death included progressive lymphoma in 15 patients and transplant-related mortality in two.
Figure 2. Overall and progression-free survival.
Overall and progression-free survival for 51 patients with relapsed lymphoma treated with gemcitabine, carboplatin, dexamethasone, and rituxumab (for CD20 + patients).
Peripheral blood stem cell mobilization
All 17 patients who attempted autologous peripheral blood stem cell PBSC mobilization with the use of filgrastim immediately following GCD(R) were successful with a median CD34 yield of 10.9 ×106/kg (range 5–24 ×106/kg). PBSC mobilization kinetics revealed day 14 to be the median day of initial apheresis with a range from day 11–19. No patients required a second mobilization attempt or received plerixafor.
Adverse events
Grade 3 and 4 hematologic toxicities were observed 10 (22%) and 39 (76%) of patients, respectively, but there were and no life-threatening bleeding or infectious complications or treatment-related deaths (Table 4). Rates of grade 4 thrombocytopenia by cycle for all patients were: 1=57%, 2=36%, 3=33%, 4=39%. For the 28 patients that received 4 cycles of therapy the rates of grade 4 thrombocytopenia for cycles 1–4 were 50%, 25%, 32%, and 39%, respectively. There were 4 (2.5%/cycle) episodes of febrile neutropenia. Grade 4 non-hematologic toxicities were observed in 2 patients consisting of severe depression and fatigue in one patient and a pulmonary embolus in a second. The most common (>10%) non-hematologic adverse events of grade 3 or higher included laboratory abnormalities (22%), cardiovascular (14%), and pain (12%). The grade 3 cardiovascular adverse events included central catheter associated thromboses (3), hypotension (3), and a vasovagal episode after removal of an apheresis catheter (1). The pain sites noted included abdominal (2), perirectal (1), chest (1), dental (1), and tumor (1). Additional details of the non-hematologic adverse events are enumerated in Table 4. Second malignancies were identified in 5 patients after treatment including two localized squamous cell carcinomas of the skin, one breast cancer, one prostate cancer, and one myelodysplastic syndrome.
Table 4.
Patients with hematologic and non-hematologic adverse events ≥ grade 3 following gemcitabine, carboplatin, dexamethasone, and rituximab (CTC 2.0)
| Hematologic category | Grade 3 | Grade 4 |
|---|---|---|
| Neutrophils | 17 | 21 |
| Hemoglobin | 17 | 1 |
| Hemorrhage | 5 | 0 |
| Infection | 12 | 0 |
| Platelets | 10 | 35 |
| Total patients with heme toxicity | 10 | 39 |
| Non-hematologic category | Grade 3 | Grade 4 |
| Allergy/Immunology | 2 | 0 |
| Cardiovascular | 6 | 1 |
| Constitutional | 4 | 1 |
| Gastrointestinal | 4 | 0 |
| Metabolic/Laboratory | 11 | 0 |
| Nausea | 2 | 0 |
| Neurology | 2 | 1 |
| Pain | 6 | 0 |
| Pulmonary | 3 | 0 |
| Total patients with non-heme toxicity | 23 | 2 |
Discussion
In this trial we examined the safety and potential efficacy of GCD(R) as an outpatient regimen for a broad variety of lymphoma histologies. Not surprisingly, we observed significant activity of this regimen in the 14 patients with HL, wherein the single-agent response rate with gemcitabine is reported to be approximately 40%13. Importantly there were no toxic deaths with this regimen, no related life-threatening complications, and significant non-hematologic adverse events were uncommon. Despite the moderate toxicity profile and the encouraging observed response rates, we acknowledge that larger more appropriately powered disease-specific studies are necessary to more carefully characterize the efficacy of this regimen.
One important consideration when administering salvage chemotherapy is the ability of the regimen to effectively mobilize PBSC. In this study, all patients who attempted mobilization with GCD(R) were successful with a median CD34 yield of 10.9 ×106/kg (range 5–24 ×106/kg). These results compare favorably to published results with the traditional regimens of RICE (88% success rate, median 6.3 × 106/kg, range, 0–15.6 × 106/kg) and DHAP (95% success rate, median 13 × 106/kg, range, 0.9–85.1 × 106/kg) 1,31. Other gemcitabine-based regimens have also demonstrated effective PBSC yields and along with our data indicate that this agent can be readily employed prior to autologous harvest 16,18. These data highlight the importance of evaluating the impact on stem cell reserve and PBSC mobilization potential when incorporating newer agents into regimens for transplant-eligible patients7,10,11,32.
Formal comparisons of GCD(R)’s efficacy with more commonly employed regimens are problematic due to potential imbalances in patient selection. For example, patients in our study had received more prior therapy (median 2 prior regimens), were older (median age 58), and had more chemoresistant disease (62%) than those in most other salvage regimen studies 1,2,4,5,33. Despite these differences, the observed response rate was similar to more traditional strategies for comparable histologies. We expect that the high-risk features of the patients in this study may be more representative of the general population of relapsed lymphoma in the community. This feature is reflected by the fact that half of the accrual and treatment occurred at 14 community sites within the Puget Sound Oncology Consortium.
Other groups have also evaluated gemcitabine-platinum combinations in lymphoma. Crump and colleagues achieved a 53% response rate using gemcitabine, dexamethasone and cisplatin (GDP) in patients with relapsed aggressive lymphoma who had received predominantly one prior therapy, though 6% experienced a toxic death from this regimen19. Similar efficacy with GDP was observed by this group in HL with overall responses occurring in 70% of patients16. Though this trial was designed at a time when there was little published data on the efficacy of oxaliplatin in lymphoma, others have since evaluated the combination of gemcitabine and oxaliplatin, also demonstrating clinical activity in MCL and DLBCL 20,34. Our data contribute to this overall literature as the first multi-center prospective study to evaluate the safety and efficacy of a gemcitabine-carboplatin combination across a variety of relapsed lymphoid histologies. Together these data suggest that all platinum analogs are effective and the specific choice can be determined by the most relevant side effect profile for a given patient35.
This prospective, multi-center trial suggests that GCD(R) is a safe regimen, and we consider the response rates observed across a wide age range of patients and a broad variety of heavily pretreated lymphoma histologies to be encouraging. Refinements in more traditional chemotherapeutic strategies such as these may continue to contribute to improvements in the lives of those suffering from lymphoma.
Acknowledgments
Research Support: Eli Lilly Incorporated, NIH grants P01CA44991, R01CA76287, R01CA109663, K23CA85479, K08CA095448, Lymphoma Research Foundation Mantle Cell Lymphoma Research Initiative, SCOR Grant 7040 from the Leukemia and Lymphoma Society, the Mary A. Wright Memorial Research Fund, and a donation from Frank and Betty Vandermeer. AKG is a Scholar in Clinical Research of the Leukemia and Lymphoma Society.
Special thanks to Tina Bogne, Mark Brockman, Britt Kammerer, Nancy Knudsen, RN, Jennifer Roden, Greg Whitman, the additional PSOC investigators who contributed to this study (Drs. Albert Brady, Eric Chen, Vijajayakrishna Gadi, Robert Gersh, John Hansen, Peter Jiang, Theodore Kim, Kirk Lund, Sareena Malhi, David Maloney, Daniel Martin, Robert McCroskey, Vivian Oehler, Shushma Pant, Nanette Robinson, William Rubin, Peter Schelgel, Gerald Segal, Howard West, and Robert Witham), and the patients who participated.
Footnotes
Presented in part at the 2008 Meeting of the American Society of Hematology, San Francisco, CA.
Authorship Contributions: Ajay K. Gopal: Designed and ran the study, contributed patients, analyzed data, wrote the manuscript.
Oliver W. Press: Assisted in the design of the study, contributed patients, reviewed data, edited the manuscript.
Andrei R. Shustov: Contributed patients, reviewed data, edited the manuscript.
Stephen H. Petersdorf: Contributed patients, reviewed data, edited the manuscript.
Ted A. Gooley: Assisted in the design of the study, analyzed data, edited the manuscript.
Jasmine T. Daniels: Contributed patients, reviewed data, edited the manuscript.
Mitchell A. Garrison: Contributed patients, reviewed data, edited the manuscript.
George F. Gjerset: Contributed patients, reviewed data, edited the manuscript.
Matthew Lonergan: Contributed patients, reviewed data, edited the manuscript.
Anne E. Murphy: Contributed patients, reviewed data, edited the manuscript.
Julie C. Smith: Contributed patients, reviewed data, edited the manuscript.
John M. Pagel: Assisted in the design of the study, contributed patients, reviewed data, edited the manuscript.
Conflict of Interest Disclosure: Ajay K. Gopal: Eli Lilly-Research Funding
Others: None
References
- 1.Kewalramani T, Zelenetz AD, Nimer SD, et al. Rituximab and ICE as second-line therapy before autologous stem cell transplantation for relapsed or primary refractory diffuse large B-cell lymphoma. Blood. 2004;103:3684–3688. doi: 10.1182/blood-2003-11-3911. [DOI] [PubMed] [Google Scholar]
- 2.Press OW, Livingston R, Mortimer J, Collins C, Appelbaum F. Treatment of relapsed non-Hodgkin’s lymphomas with dexamethasone, high- dose cytarabine, and cisplatin before marrow transplantation. J Clin Oncol. 1991;9:423–431. doi: 10.1200/JCO.1991.9.3.423. [DOI] [PubMed] [Google Scholar]
- 3.Moskowitz CH, Kewalramani T, Nimer SD, Gonzalez M, Zelenetz AD, Yahalom J. Effectiveness of high dose chemoradiotherapy and autologous stem cell transplantation for patients with biopsy-proven primary refractory Hodgkin’s disease. Br J Haematol. 2004;124:645–652. doi: 10.1111/j.1365-2141.2003.04828.x. [DOI] [PubMed] [Google Scholar]
- 4.Velasquez WS, McLaughlin P, Tucker S, et al. ESHAP--an effective chemotherapy regimen in refractory and relapsing lymphoma: a 4-year follow-up study. J Clin Oncol. 1994;12:1169–1176. doi: 10.1200/JCO.1994.12.6.1169. [DOI] [PubMed] [Google Scholar]
- 5.Velasquez WS, Cabanillas F, Salvador P, et al. Effective salvage therapy for lymphoma with cisplatin in combination with high-dose Ara-C and dexamethasone (DHAP) Blood. 1988;71:117–122. [PubMed] [Google Scholar]
- 6.Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 7.Witzig TE, Geyer SM, Ghobrial I, et al. Phase II trial of single-agent temsirolimus (CCI-779) for relapsed mantle cell lymphoma. J Clin Oncol. 2005;23:5347–5356. doi: 10.1200/JCO.2005.13.466. [DOI] [PubMed] [Google Scholar]
- 8.Witzig TE, White CA, Gordon LI, et al. Safety of yttrium-90 ibritumomab tiuxetan radioimmunotherapy for relapsed low-grade, follicular, or transformed non-hodgkin’s lymphoma. J Clin Oncol. 2003;21:1263–1270. doi: 10.1200/JCO.2003.08.043. [DOI] [PubMed] [Google Scholar]
- 9.Kaminski MS, Zelenetz AD, Press OW, et al. Pivotal study of iodine I 131 tositumomab for chemotherapy-refractory low-grade or transformed low-grade B-cell non-Hodgkin’s lymphomas. J Clin Oncol. 2001;19:3918–3928. doi: 10.1200/JCO.2001.19.19.3918. [DOI] [PubMed] [Google Scholar]
- 10.Wiernik PH, Lossos IS, Tuscano JM, et al. Lenalidomide monotherapy in relapsed or refractory aggressive non-Hodgkin’s lymphoma. J Clin Oncol. 2008;26:4952–4957. doi: 10.1200/JCO.2007.15.3429. [DOI] [PubMed] [Google Scholar]
- 11.Friedberg JW, Cohen P, Chen L, et al. Bendamustine in patients with rituximab-refractory indolent and transformed non-Hodgkin’s lymphoma: results from a phase II multicenter, single-agent study. J Clin Oncol. 2008;26:204–210. doi: 10.1200/JCO.2007.12.5070. [DOI] [PubMed] [Google Scholar]
- 12.Haperen VRV. 2′2′ Difluurodeoxycytidine (gemcitabine) incorporation into DNA and RNA of tumor cell lines. Biochem Pharmachol. 1993;46:762–766. doi: 10.1016/0006-2952(93)90566-f. [DOI] [PubMed] [Google Scholar]
- 13.Santoro A, Bredenfeld H, Devizzi L, et al. Gemcitabine in the treatment of refractory Hodgkin’s disease: results of a multicenter phase II study. J Clin Oncol. 2000;18:2615–2619. doi: 10.1200/JCO.2000.18.13.2615. [DOI] [PubMed] [Google Scholar]
- 14.Zinzani PL, Baliva G, Magagnoli M, et al. Gemcitabine treatment in pretreated cutaneous T-cell lymphoma: experience in 44 patients. J Clin Oncol. 2000;18:2603–2606. doi: 10.1200/JCO.2000.18.13.2603. [DOI] [PubMed] [Google Scholar]
- 15.Fossa A, Santoro A, Hiddemann W, et al. Gemcitabine as a single agent in the treatment of relapsed or refractory aggressive non-Hodgkin’s lymphoma. J Clin Oncol. 1999;17:3786–3792. doi: 10.1200/JCO.1999.17.12.3786. [DOI] [PubMed] [Google Scholar]
- 16.Baetz T, Belch A, Couban S, et al. Gemcitabine, dexamethasone and cisplatin is an active and non-toxic chemotherapy regimen in relapsed or refractory Hodgkin’s disease: a phase II study by the National Cancer Institute of Canada Clinical Trials Group. Ann Oncol. 2003;14:1762–1767. doi: 10.1093/annonc/mdg496. [DOI] [PubMed] [Google Scholar]
- 17.Bartlett NL, Niedzwiecki D, Johnson JL, et al. Gemcitabine, vinorelbine, and pegylated liposomal doxorubicin (GVD), a salvage regimen in relapsed Hodgkin’s lymphoma: CALGB 59804. Ann Oncol. 2007;18:1071–1079. doi: 10.1093/annonc/mdm090. [DOI] [PubMed] [Google Scholar]
- 18.Corazzelli G, Russo F, Capobianco G, Marcacci G, Della Cioppa P, Pinto A. Gemcitabine, ifosfamide, oxaliplatin and rituximab (R-GIFOX), a new effective cytoreductive/mobilizing salvage regimen for relapsed and refractory aggressive non-Hodgkin’s lymphoma: results of a pilot study. Ann Oncol. 2006;17(Suppl 4):iv18–24. doi: 10.1093/annonc/mdj994. [DOI] [PubMed] [Google Scholar]
- 19.Crump M, Baetz T, Couban S, et al. Gemcitabine, dexamethasone, and cisplatin in patients with recurrent or refractory aggressive histology B-cell non-Hodgkin lymphoma: a Phase II study by the National Cancer Institute of Canada Clinical Trials Group (NCIC-CTG) Cancer. 2004;101:1835–1842. doi: 10.1002/cncr.20587. [DOI] [PubMed] [Google Scholar]
- 20.El Gnaoui T, Dupuis J, Belhadj K, et al. Rituximab, gemcitabine and oxaliplatin: an effective salvage regimen for patients with relapsed or refractory B-cell lymphoma not candidates for high-dose therapy. Ann Oncol. 2007;18:1363–1368. doi: 10.1093/annonc/mdm133. [DOI] [PubMed] [Google Scholar]
- 21.Cole PD, Schwartz CL, Drachtman RA, de Alarcon PA, Chen L, Trippett TM. Phase II study of weekly gemcitabine and vinorelbine for children with recurrent or refractory Hodgkin’s disease: a children’s oncology group report. J Clin Oncol. 2009;27:1456–1461. doi: 10.1200/JCO.2008.20.3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iaffaioli RV, Tortoriello A, Facchini G, et al. Phase I-II study of gemcitabine and carboplatin in stage IIIB-IV non- small-cell lung cancer. J Clin Oncol. 1999;17:921–926. doi: 10.1200/JCO.1999.17.3.921. [DOI] [PubMed] [Google Scholar]
- 23.Gandara DR, Lau DH, Lara PN, Jr, Edelman MJ. Gemcitabine/carboplatin combination regimens: importance of dose schedule. Oncology (Huntingt) 2000;14:26–30. [PubMed] [Google Scholar]
- 24.Yardley DA, Burris HA, 3rd, Simons L, et al. A phase II trial of gemcitabine/carboplatin with or without trastuzumab in the first-line treatment of patients with metastatic breast cancer. Clin Breast Cancer. 2008;8:425–431. doi: 10.3816/CBC.2008.n.051. [DOI] [PubMed] [Google Scholar]
- 25.Pfisterer J, Plante M, Vergote I, et al. Gemcitabine plus carboplatin compared with carboplatin in patients with platinum-sensitive recurrent ovarian cancer: an intergroup trial of the AGO-OVAR, the NCIC CTG, and the EORTC GCG. J Clin Oncol. 2006;24:4699–4707. doi: 10.1200/JCO.2006.06.0913. [DOI] [PubMed] [Google Scholar]
- 26.Ng EW, Sandler AB, Robinson L, Einhorn LH. A phase II study of carboplatin plus gemcitabine in advanced non-small- cell lung cancer (NSCLC): a hoosier oncology group study. Am J Clin Oncol. 1999;22:550–553. doi: 10.1097/00000421-199912000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Harris NL, Jaffe ES, Diebold J, Flandrin G, Muller-Hermelink HK, Vardiman J. Lymphoma classification--from controversy to consensus: the R.E.A.L. and WHO Classification of lymphoid neoplasms. Ann Oncol. 2000;11 (Suppl 1):3–10. [PubMed] [Google Scholar]
- 28.Swerdlow SH, Campo E, Harris NL, et al., editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC Press; 2008. [Google Scholar]
- 29.Cheson BD, Horning SJ, Coiffier B, et al. Report of an International Workshop to Standardize Response Criteria for Non-Hodgkin’s Lymphomas. J Clin Oncol. 1999;17:1244–1253. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 30.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 31.Smardova L, Engert A, Haverkamp H, et al. Successful mobilization of peripheral blood stem cells with the DHAP regimen (dexamethasone cytarabine cisplatinum) plus granulocyte colony-stimulating factor in patients with relapsed Hodgkin’s disease. Leuk Lymphoma. 2005;46:1017–1022. doi: 10.1080/10428190500064276. [DOI] [PubMed] [Google Scholar]
- 32.Duvic M, Talpur R, Ni X, et al. Phase 2 trial of oral vorinostat (suberoylanilide hydroxamic acid, SAHA) for refractory cutaneous T-cell lymphoma (CTCL) Blood. 2007;109:31–39. doi: 10.1182/blood-2006-06-025999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moskowitz CH, Nimer SD, Zelenetz AD, et al. A 2-step comprehensive high-dose chemoradiotherapy second-line program for relapsed and refractory Hodgkin disease: analysis by intent to treat and development of a prognostic model. Blood. 2001;97:616–623. doi: 10.1182/blood.v97.3.616. [DOI] [PubMed] [Google Scholar]
- 34.Rodriguez J, Gutierrez A, Palacios A, et al. Rituximab, gemcitabine and oxaliplatin: an effective regimen in patients with refractory and relapsing mantle cell lymphoma. Leuk Lymphoma. 2007;48:2172–2178. doi: 10.1080/10428190701618268. [DOI] [PubMed] [Google Scholar]
- 35.Markman M. Toxicities of the platinum antineoplastic agents. Expert Opin Drug Saf. 2003;2:597–607. doi: 10.1517/14740338.2.6.597. [DOI] [PubMed] [Google Scholar]