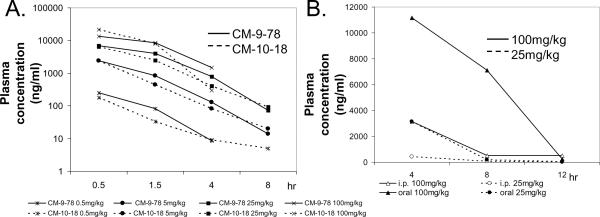
Fig. 2. Pharmacokinetic properties of CM-9-78 and CM-10-18.
(A) Dose escalation studies of CM-9-78 and CM-10-18. Escalating doses, as indicated, were given to rats every 48 to 72 hours, via i.p. route. Blood samples were harvested at 0.5, 1.5, 4 and 8 (except at dose 100mg/kg) hours after injection to measure the plasma concentration (ng/ml) of the test compounds. Each data points represent average values obtained from 3 rats. (B) Single dose administration via oral gavage or i.p. injection of CM-10-18 in mice. CM-10-18 was given at 25mg/kg or 100mg/kg. Blood samples were drawn at 4, 8, and 12 hours post administration to measure the plasma concentration of compound (ng/ml). Each data points represent average values obtained from 2 mice.