Abstract
A survey of mammalian genomes has unexpectedly unearthed DNA derived from bornaviruses, leading to speculation about the role of these viruses in causing mutations with evolutionary and medical consequences.
Some people might find it disquieting that a hefty 8% of human genetic material originates not from our vertebrate ancestors but from viruses. The assimilation of viral sequences into the host genome is a process referred to as endogenization. It occurs when viral DNA integrates into a chromosome of reproductive germline cells and is subsequently passed from parent to offspring. Until now, retroviruses were the only viruses known to generate such endogenous copies in vertebrates. But on page 84 of this issue, Horie et al. [1] report that non-retroviral viruses called bornaviruses have been endogenized repeatedly during mammalian evolution. The finding unveils bornaviruses as a potential cause of mutation and also as an unforeseen source of genomic innovation (Fig. 1).
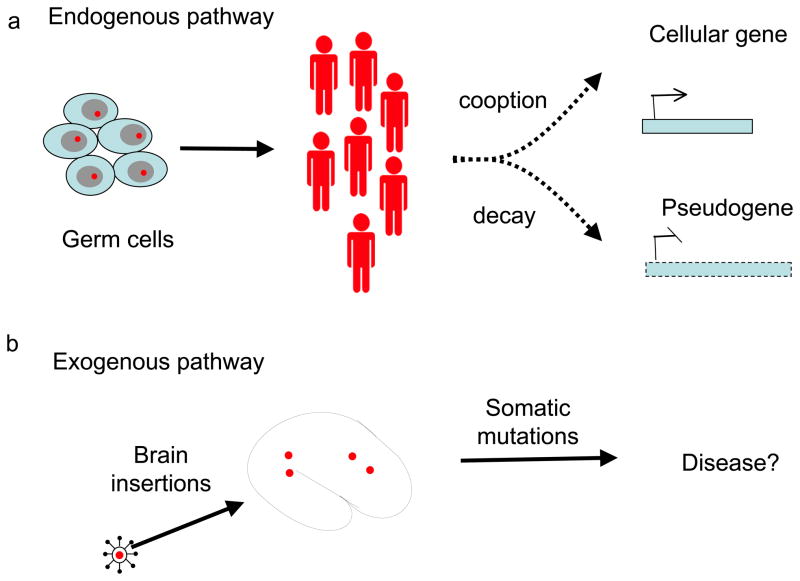
Figure 1. Bornavirus in the genome, for better or worse.
a, Horie et al. [1] report that bornavirus gene sequences (red stars) became integrated into the germline of our ancestors, and through vertical transmission (by conventional inheritance) have become ‘fixed’ in the genome, thereby becoming endogenous viral insertions. A fixed viral insertion can follow one of two evolutionary fates: it can either decay into a pseudogene or be co-opted to form a new gene whose product has a cellular function. b, Circulating bornavirus sequences can become integrated into the genome of brain cells (the current target of Borna disease virus) after infection (exogenous insertion). These sequences are not heritable, but might cause mutations that interfere with brain function and may contribute to the development of psychiatric disorders.
Borna disease virus (BDV) owes its name to the town of Borna, Germany, the site of a dreadful virus epidemic that decimated a regiment of cavalry horses in 1885. However, it is only recently that BDV has been characterized genetically: it belongs to the order Mononegavirales, and is a negative-sense RNA virus (in which the single-stranded RNA genome has the opposite sequence to messenger RNA). BDV infects a range of birds and mammals, including humans, and is unique among RNA viruses in that it naturally infects only neurons, establishing a persistent infection in its host’s brain. In addition, the entire life cycle of BDV takes place in the nucleus of the infected cells, and does not require chromosomal integration [2]. This intimate association of BDV with the cell nucleus prompted Horie et al. to investigate whether bornaviruses may have left behind a record of past infection in the form of endogenous elements.
Horie et al. searched the 234 currently available eukaryotic genomes for sequences that are similar to that of BDV, and unearthed a plethora of endogenous Borna-like N (EBLN) elements in diverse mammals. The sequences of these elements resemble the nucleoprotein (N) gene of BDV, which encodes a structural protein involved in packaging the viral RNA into a nucleo capsid [2]. The authors show1 that bornavirus endogenization has occurred in multiple mammalian lineages and at different times, ranging from more than 40 million years ago in anthropoid primates to less than 10 million years ago in squirrels. These molecular fossils add to the growing evidence [3–6] for the long-term coevolution of RNA viruses and their mammalian hosts.
All instances of endogenization described by Horie et al.1 correspond to the N gene, and although most EBLN sequences are fragmentary and seem to be non-functional (they have decayed into pseudogenes), surprisingly, two EBLNs in the human genome are annotated as protein-coding genes. They retain long open reading frames (sequences that seem to encode proteins) and are transcribed from DNA into mRNAs in the various tissues and cell lines examined [1]. Also, one of the two proteins (LOC340900) has been reported7 to interact with several well-known cellular proteins. Thus, the discovery of EBLNs uncovers two cases of viral DNA that has apparently been coopted to form cellular genes (Fig. 1a). Although it is not known whether EBLN-derived proteins are functional in human cells, these proteins may have been usurped by the host, at least initially, to serve an antiviral function. There are precedents for this in mice and sheep [8,9], in which endogenous retro viral capsid proteins offer protection against exogenous retroviral infections.
How are EBLN elements generated? Unlike retroviruses, BDV does not need to integrate into the host DNA to replicate, and therefore the virus genome does not encode the machinery for reverse transcription of its RNA into DNA. Despite this, using the polymerase chain reaction, Horie et al. [1] were able to detect BDV DNA in various infected cell lines and in the brain of persistently infected mice. Furthermore, after infecting human cells for 30 days, the authors could isolate chromosomally integrated BDV DNA along with flanking host genomic sequences. These BDV insertions resemble EBLN elements in that they are derived from the N gene and exhibit the hallmarks of retroposition (the process by which RNA is integrated into a DNA genome), including a stretch of adenine nucleotides at the 3′ end of the inserted element and a short duplication of the target site.
In mammalian genomes, retroposition is primarily driven by the activity of L1 long interspersed nucleotide elements — pieces of mobile DNA that make copies of themselves and reinsert into the genome [10]. L1 has colonized the genome of mammals for more than 100 million years and continues to replicate actively in several species, including humans. The L1 enzymatic machinery can reverse transcribe its own RNA into DNA, but can also act on non-L1 RNA templates — throughout evolution, this promiscuity has caused the bombardment of mammalian genomes with millions of DNA inserts10. The abundance of BDV RNA in the nucleus of persistently infected cells, coupled with some peculiar properties of the N gene RNA, might have promoted their fortuitous recognition by the L1 machinery.
The fact that Horie and colleagues [1] could readily detect BDV DNA and chromosomal insertions in human cells suggests that BDV retroposition might occur at an appreciable frequency during BDV infection, creating a source of mutation in infected individuals (Fig. 1b). This yields a tantalizing and testable hypothesis for the alleged, but still controversial, causative association of BDV infection with certain psychiatric disorders, such as schizophrenia and mood disorders [2,11]. This possibility becomes even more intriguing when considering the recent demonstration of L1 hyperactivity in the human brain [12], the primary site of BDV infection.
References
- 1.Horie M, et al. Nature. 2010;463:84–87. doi: 10.1038/nature08695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tomonaga K, Kobayashi T, Ikuta K. Microbes Infect. 2002;4:491–500. doi: 10.1016/s1286-4579(02)01564-2. [DOI] [PubMed] [Google Scholar]
- 3.Katzourakis A, et al. Science. 2009;325:1512. doi: 10.1126/science.1174149. [DOI] [PubMed] [Google Scholar]
- 4.Gilbert C, Maxfield DG, Goodman SM, Feschotte C. PLoS Genet. 2009;5:e1000425. doi: 10.1371/journal.pgen.1000425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gifford RJ, et al. Proc Natl Acad Sci USA. 2008;105:20362–20367. doi: 10.1073/pnas.0807873105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holmes EC. Annu Rev Microbiol. 2008;62:307–328. doi: 10.1146/annurev.micro.62.081307.162912. [DOI] [PubMed] [Google Scholar]
- 7.Ewing RM, et al. Mol Syst Biol. 2007;3:89. doi: 10.1038/msb4100134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Best S, Le Tissier P, Towers G, Stoye JP. Nature. 1996;382:826–829. doi: 10.1038/382826a0. [DOI] [PubMed] [Google Scholar]
- 9.Arnaud F, Murcia PR, Palmarini M. J Virol. 2007;81:11441–11451. doi: 10.1128/JVI.01214-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cordaux R, Batzer MA. Nature Rev Genet. 2009;10:691–703. doi: 10.1038/nrg2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rott R, et al. Science. 1985;228:755–756. doi: 10.1126/science.3922055. [DOI] [PubMed] [Google Scholar]
- 12.Coufal NG, et al. Nature. 2009;460:1127–1131. doi: 10.1038/nature08248. [DOI] [PMC free article] [PubMed] [Google Scholar]