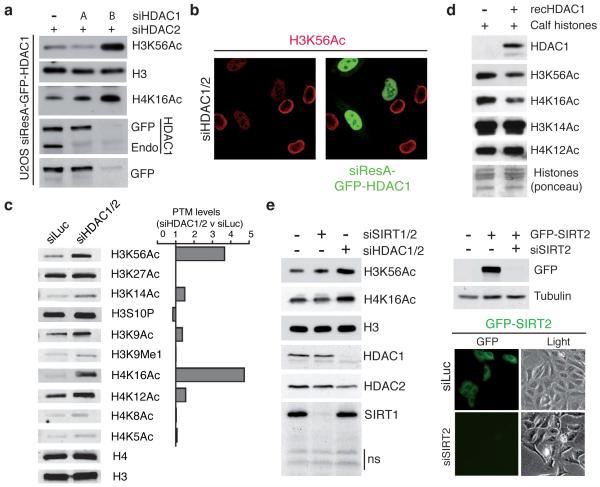
Figure 4.
HDAC1 and HDAC2 specifically and directly target H3K56 and H4K16 (a) siResistant HDAC1 (siResA-GFP-HDAC1) rescues defects of H3K56 and H4K16 hyper-acetylation in siHDAC1/2 U2OS cells. (see methods for details; Endo = endogenous protein). (b) U2OS cells expressing siResA-GFP-HDAC1 rescues H3K56 hyper-acetylation in cells depleted for endogenous HDAC1/2. Cells were treated with the indicated siRNAs and analyzed by IF with a H3K56Ac antibody (Epitomics). (c) HDAC1/2-depleted U2OS cells exhibit H3K56 and H4K16 hyper-acetylation. Quantification of samples in a was performed with the LI-COR Odyssey infrared imaging system; ratios of signals in siHDAC1/2 versus siLuc are given and histone-modification levels were normalized to histone H3 or H4 levels. (d) Recombinant HDAC1 deacetylates H3K56 and H4K16 in vitro. (e) Depletion of SIRT1/2 does not increase H3K56Ac and H4K16Ac levels in HeLa cells. Experiments were performed as in a. siRNA sequences targeting SIRT1 and SIRT2 were obtained from a previous study22. Lower right panel confirms the documented cytoplasmic localization of SIRT2 and the efficient depletion of GFP-SIRT2 by fluorescence microscopy.