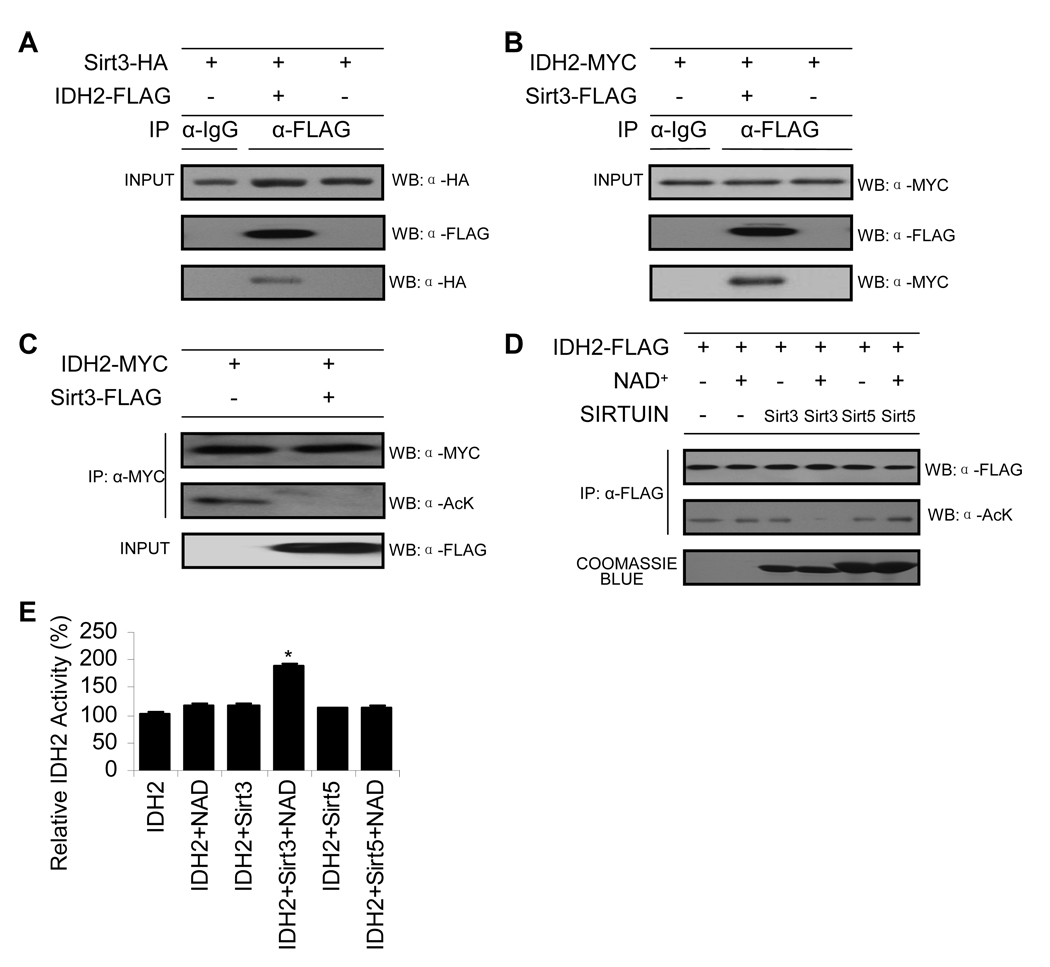
Figure 5. Sirt3 Directly Deacetylates Idh2 and Stimulates Activity.
(A–B) Sirt3 interacts with Idh2. Idh2 or Sirt3 were immunoprecipitated from HEK293 cell lysates with IgG antibody or FLAG beads. Precipitated Idh2-FLAG was detected by anti-FLAG antibody and co-IP Sirt3-HA was detected by anti-HA as indicated (A). Precipitated Sirt3-FLAG was detected by anti-FLAG antibody and co-IP Idh2-MYC was detected by anti-MYC as indicated (B) (n = 3).
(C) Sirt3 deacetylates Idh2 in HEK293 cells. Idh2 was cotransfected with or without Sirt3, isolated by immunoprecipitation with anti-MYC antibody followed by western blotting with anti-acetyl-lysine antibody (n = 3).
(D) Sirt3, but not Sirt5, deacetylates Idh2 in vitro. Acetylated Idh2 was prepared as outlined in Experimental Procedure, and was incubated with purified recombinant Sirt3 or Sirt5 with or without NAD+ at 37 °C for 1 hour. Acetylation status was assessed by western blotting with anti-acetyl-lysine antibody (n = 3). An anti-FLAG western shows equivalent Idh2 protein levels were used and coomassie staining shows purified Sirt3 and Sirt5.
(E) In vitro deacetylation of Idh2 by Sirt3, but not Sirt5, stimulates Idh2 activity. Acetylated Idh2 in buffer (Tris (pH 7.5), with or without 1 mM NAD, and 1 mM DTT) was incubated with purified 50 nM Sirt3 or Sirt5 (Hallows et al., 2006) at 37°C for 1 hour, followed by Idh2 activity assay (n = 3). *Significantly different from Idh2 alone (P < 0.05). Data are means ± SEM. See also Figure S4.