Experiments of small bowel or multivisceral transplantation in rodents have established the potency of FK 506 in preventing intestinal rejection. 1–4 We present here observations with the more difficult model of canine small bowel transplantation.
MATERIALS AND METHODS
Adult mongrel dogs of both sexes, weighing 12.5 to 29.0 kg, were used. Under anesthesia with isoflurane, the entire small bowel except the duodenum and 5 cm of the terminal ileum was isolated with its vascular pedicle, and excised. The graft was perfused on the back table with cold lactated Ringer’s solution containing 2500 U/L of heparin. The transplantation technique was essentially the same as that originally described by Lellihei et al,5 in which the grafts were exchanged between the donor and the recipient. The superior mesenteric artery and the superior mesenteric vein were anastomosed end-to-end. A 10-cm segment of graft-distal ileum was exteriorized, as a Thiry-Vella loop, for visual monitoring and postoperative mucosal biopsies.
The animals were divided into nine groups, according to treatment (Table 1). Thirty-four (33%) of the original 103 animals who died within 5 days from various technical causes were excluded from the final analysis. Except for the intramuscular (IM) group (B), treatment was continued daily until animals died or were killed with an anesthesia injection when they became lethargic or lost more than 40% of their preoperative body weight. Control animals with autotransplantation were killed 1 year postoperatively.
Table 1.
Experimental Groups
| Route | Group | n | FK 506 (mg/kg per day) |
Graft |
|---|---|---|---|---|
| IV | A-1 | 8 | 0.00 | Auto |
| A-2 | 5 | 0.20 | Auto | |
| A-3 | 6 | 0.00 | Allo | |
| A-4 | 7 | 0.05 | Allo | |
| A-5 | 6 | 0.075 | Allo | |
| A-6 | 11 | 0.10 | Allo | |
| A-7 | 8 | 0.20 | Allo | |
| IM* | B | 7 | 1.00 | Allo |
| Oral | C | 11 | 1.50 | Allo |
Intramuscular FK 506 (1 mg/kg per day) was given on postoperative days 3, 4, and 5.
Posttransplant observations were of the clinical course, stools, and appearance of the isolated segment stoma. Measurements were of body weight, electrolytes, and blood chemistries. FK 506 plasma levels were monitored by a standardized method of enzyme immunoassay.6 Maltose absorption test (0.5 mg/kg) was performed periodically using the modified method of Billiar et al. 7
Tissues obtained from stomal biopsies or at postmortem examinations were fixed with 10% buffered formalin and stained with hematoxylin-eosin. Histological features of acute rejection were similar to those reported in treated4 or untreated,8 rats and included hemorrhagic mucosal necrosis, lymphocytic infiltration of the lamina propria, epithelial damage, and lacteal dilatation. IM and perineural mononuclear cell infiltrate, obliterative arteritis, and thickening of the muscular layer with accompanying mucosal alterations including villous atrophy were features of chronic rejection.
RESULTS
Autotransplantation
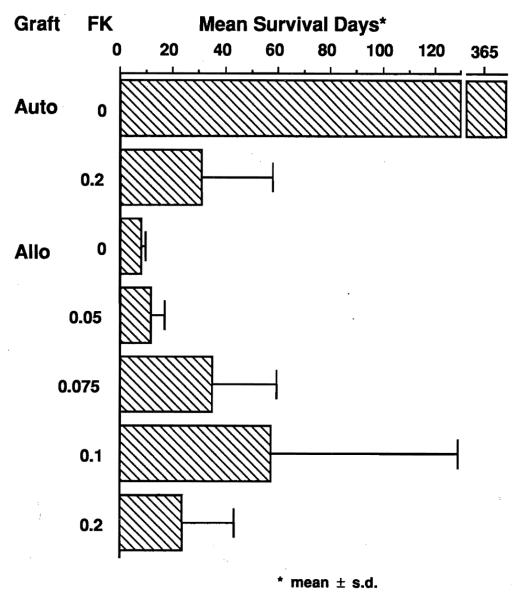
Untreated dogs with autografts (A-1) lived indefinitely. Mean survival after autotransplantation was reduced to 31 days when 12 mg/kg IV FK 506 was given (Fig 1). The usual cause of death was lethal emaciation.
Fig 1.
Mean survival days of the animals treated with IV FK 506. FK 506 (mg/kg per day) was given daily until animals died or were sacrificed. Autotransplant control animals (no FK 506) were sacrificed at 1 year.
Allotransplantation
In contrast, untreated recipients of allografts (A-3) died of rejection within 10 days (mean 7.8). Mean survival of 57.5 days was obtained in dogs treated with 0.1 mg/kg per day IV FK 506 (A-6) or with 0.075 mg/kg per day (A-5) (Fig 1). When the IV dose was increased to 0.2 mg/kg per day (A-7), mean survival was reduced to 23.3 days.
Mean survival of the animals treated with 1 mg/kg IM FK 506 on postoperative days 3, 4, and 5 only (group B) was 17.7 days (Fig 1). Survival using daily oral treatment with 1.5 mg/kg (group C) was 22.8 days.
In untreated recipients, or those with the lowest dose of FK 506, the cause of death was always acute rejection with hemorrhagic necrosis of the mucosa. At the IV dose of 0.1 mg/kg per day, half of the animals died, either of acute rejection or a combination of acute or chronic rejection. In animals dying after 1 month, many of the grafts had hypertrophic thickening of the muscular layer and neuronal hypertrophy. These findings were not present in any of the intestinal autografts which, in untreated animals, were sampled at a much later time.
At the highest IV FK 506 dose, one-fourth of the dogs died of rejection, and the remaining animals died of lethal emaciation. Clinical or histological graft-versus-host disease (GVHD) was not seen in any of these animals.
Absorption
Preoperatively, and 1 week after transplantation (Fig 2), elevations of blood glucose after oral maltose administration were not different in the autograft group (A-1) compared to the allograft groups with (A-6) or without FK 506 treatment (A-3). Later postoperative studies were not systematically obtained.
Fig 2.
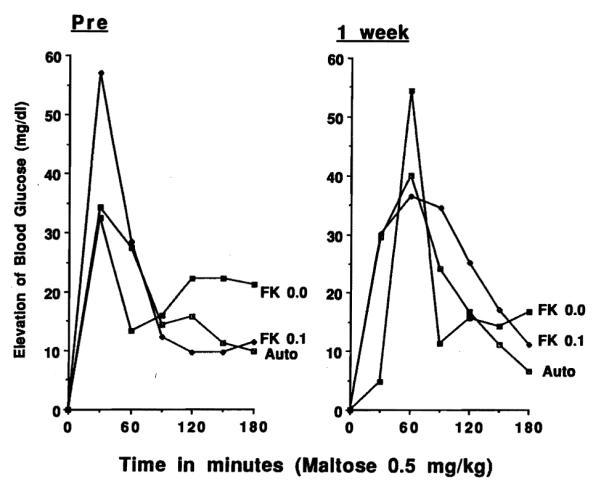
Maltose absorption test before intestinal transplantation (PRE) and 1 week postoperatively in animals receiving autografts without treatment, or allografts with or without 0.1 mg/kg per day IV FK 506. Net increases of mean blood glucose levels (mg/dL) after 0.5 mg/kg of maltose administration are shown.
Plasma Levels of FK 506
Five days after transplantation, plasma concentrations varied according to the route of administration. In the IV treatment groups, plasma FK 506 levels were between 0.15 and 0.24 ng/mL at doses from 0.05 to 0.1 mg/kg per day. The highest FK 506 dose of 0.2 mg/kg per day gave 1.56 ng/mL as a mean trough level at 5 days. Mean FK 506 plasma levels were 1.0 ng/mL and 1.04 ng/mL in the IM group (group B) and in the oral group (group C), respectively.
DISCUSSION
Early research on small bowel transplantation in isolation5 or as part of multivisceral grafts9 was with the nonimmunosuppressed dog. Many subsequent studies have been performed in dogs to evaluate the immunosuppressive agents in bowel transplantation, 10–12 histopathologic changes,13,14 and the absorptive function of allografts. 15 The present study is the first in dogs to assess the efficacy of FK 506 for small bowel transplantation. The results were comparable to those previously reported with Cy A. 12 FK 506 significantly prolonged animal survival, especially at IV doses of 0.075 and 0.1 mg/kg per day. The maximum survival was 258 days with the latter dose. Higher doses caused lethal emaciation similar to that reported in dogs in canine renal and hepatic transplantation experiments. 16–18
Although we have concluded that the dog is a difficult if not inappropriate species for most transplant studies with FK 506, the wealth of information in past canine experiments with the bowel as well as other transplanted organs prompted this study. The high mortality, as in our earlier studies of kidney16,17 and liver transplantation,18 was partly ascribable to drug toxicity, which precluded doses high enough to consistently prevent rejection. Rejection appeared more difficult to control than with the canine kidney or liver with which long survival could be achieved with a short course of therapy. 16 The intestinal recipients of the present study had little benefit from a 3-day IM course on days 3 to 5. As in rat experiments4 and in our recent human experience,19 there was no evidence of the GVHD that was first seen in untreated canine multivisceral recipients,20 and subsequently precisely defined in rats by Monchik and Russell.21
It is debatable if mucosal biopsies for the monitoring of graft rejection can be used to guide management. In our dogs, moderate to severe rejection could be diagnosed by this method during the second to fourth postoperative week, but the diagnostic changes preceded death by only 2 to 3 days in untreated controls and by 5 to 7 days in the treated groups. This may be too late for effective therapeutic intervention. Moreover, biopsies were unreliable during both the very early postoperative period and after 1 month because of nonspecific mucosal changes caused by surgical insults, bacterial overgrowth, mucosal atrophy, and other artifacts. Finally, for the diagnosis of chronic rejection, full thickness biopsy is required since histological changes were largely confined to the muscle layers. The procurement of such specimens will be dangerous.
It is probable that absorption tests also will be nondiscriminating. Murase et al showed, in rats, that maltose absorption was a poor predictor of pathologic changes.4 Our present study showed the intestinal graft regained absorptive function at an early period, even in untreated animals undergoing acute rejection, as estimated with the enteric maltose test.
The good early absorption of FK 506 in animals and in our human intestinal recipients 19 is in contrast to our experience in a patient with multivisceral abdominal transplantation who had poor absorption of CyA throughout 6 months of survival. 22 Our tentative opinion, based on our early experience, is that oral administration of FK 506 will be more practical than with CyA and can be depended upon soon after resumption of a diet following clinical small bowel transplantation.
CONCLUSION
IV FK 506 at doses of 0.075 and 0.1 mg/kg per day significantly prolonged survival of mongrel dogs after small bowel transplantation. A higher FK 506 dose caused lethal emaciation in both allograft and autograft recipients. Acute rejection of a moderate-to-severe grade could be diagnosed by mucosal biopsy, during the first month. Full-thickness biopsy was necessary for the diagnosis of chronic rejection. No clinical or histopathologic evidence of GVHD was observed under FK 506, and the failure to achieve better results was largely explained by incomplete control of rejection and drug toxicity. Maltose and FK 506 were absorbed efficiently during the first week after small bowel transplantation.
Acknowledgments
Supported by research grants from the Veterans Administration and Project Grant No. DK29962 from the National Institutes of Health, Bethesda, Maryland.
REFERENCES
- 1.Hoffman A, Makowka L, Banner B, et al. Transplantation. 1990;49:483. doi: 10.1097/00007890-199003000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee K, Stangl M, Todo S, et al. Transplant Proc. 1990;22:78. [PMC free article] [PubMed] [Google Scholar]
- 3.Murase N, Kim D, Todo S, et al. Transplant Proc. 1990;22:74. [PMC free article] [PubMed] [Google Scholar]
- 4.Murase N, Demetris A, Matsuzaki T, et al. Surgery. 1991;110:87. [PMC free article] [PubMed] [Google Scholar]
- 5.Lillehei RC, Goott B, Miller FA. Ann Surg. 1959;150:543. doi: 10.1097/00000658-195910000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tamura K, Kobayashi M, Hashimoto K, et al. Transplant Proc. 1987;19:23. [PubMed] [Google Scholar]
- 7.Billiar TR, Garberoglio E, Schraut WH. J Surg Res. 1984;37:75. doi: 10.1016/0022-4804(84)90164-1. [DOI] [PubMed] [Google Scholar]
- 8.Murase N, Demetris AJ, Kim DG, et al. Surgery. 1990;108:880. [PMC free article] [PubMed] [Google Scholar]
- 9.Starzl T, Kaupp H., Jr Surg Forum. 1960;11:28. [PMC free article] [PubMed] [Google Scholar]
- 10.Taylor R, Watson J, Walker F, et al. Brit J Surg. 1966;53:134. doi: 10.1002/bjs.1800530212. [DOI] [PubMed] [Google Scholar]
- 11.Lillehei RC, Idezuki Y, Feemster JA, et al. Surgery. 1967;62:721. [PubMed] [Google Scholar]
- 12.Fujiwara H, Grogan J, Raju S. Transplantation. 1987;44:469. doi: 10.1097/00007890-198710000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Millard P, Dennison A, Hughes DA, et al. Brit J Exp Pathol. 1986;67:687. [PMC free article] [PubMed] [Google Scholar]
- 14.Banner B, Hoffman A, Cai X, et al. Am J Surg Pathol. 1990;14(suppl 1):109. [PubMed] [Google Scholar]
- 15.Diliz-Perez H, McClure J, Bedetti C, et al. Transplantation. 1984;37:126. doi: 10.1097/00007890-198402000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Todo S, Ueda Y, Demetris A, et al. Surgery. 1988;104:239. [PMC free article] [PubMed] [Google Scholar]
- 17.Todo S, Demetris AJ, Ueda Y, et al. Transplant Proc. 1987;19(suppl 6):57. [PMC free article] [PubMed] [Google Scholar]
- 18.Todo S, Podesta L, ChapChap P, et al. Transplant Proc. 1987;19(suppl 6):64. [PMC free article] [PubMed] [Google Scholar]
- 19.Todo S, Tzakis AG, Abu-Elmagd K, et al. Transplantation. (in press) [Google Scholar]
- 20.Starzl TE, Kaupp HA, Jr, Brock DR, et al. Am J Surg. 1962;103:219. doi: 10.1016/0002-9610(62)90491-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monchik G, Russell P. Surgery. 1971;70:693. [PubMed] [Google Scholar]
- 22.Starzl TE, Rowe M, Todo S, et al. JAMA. 1989;26:1449. [PMC free article] [PubMed] [Google Scholar]