Figure 13.
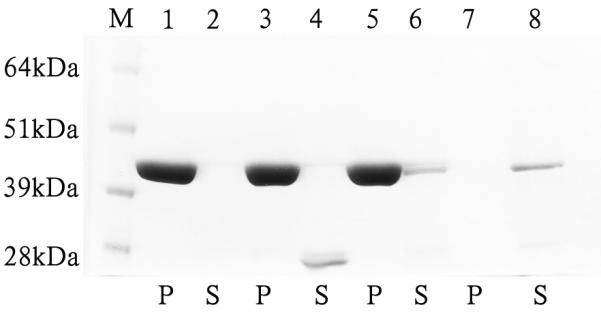
Analysis of interaction of midArgBP2-GST (Arg-15) with F-actin. Amido Black stained 6-12% gel of pellet (p) and supernatant (s) factions resulting from centrifugation of mixtures of actin filaments and midArg15-GST. Lane M: Molecular weight marker, lane 1: pellet from the tube containing actin only; lane 2: supernatant of actin alone; lane 3: pellet of actin from tube containing actin + free GST protein; lane 4: supernatant from the tube containing GST+actin. Note that no GST was detected in lane 3 indicating that GST alone does not bind F-actin. Lane 5: pellet from the reaction tube containing GST-Arg-15; lane 6: supernatant from the tube containing actin + GST-Arg-15; lane 7: pellet of the reaction tube containing GST-Arg-15 protein alone; and lane 8: supernatant from the reaction tube containing only GST-Arg-15. No staining was observed in the pellet from the reaction tube containing GST-Arg-15 suggesting that the GST-Arg-15 alone does not aggregate or precipitated that can be sedimented by ultracentrifugation. The supernatant ~45 kDa bands in lanes 6 and 8 were quantified by using NIH Image-J software and it was found that the intensity of the band in lane 6 from the reaction tube containing both F-actin and GST-Arg-15 is always lower compared to the band in lane 8 (supernatant from the reaction tube containing only GST-Arg-15 fusion protein) although the tubes were treated in an identical way. Pelleted F-actin has lowered the amount of Arg-15 in lane (6) by 33% of Arg-15 in lane 8.
