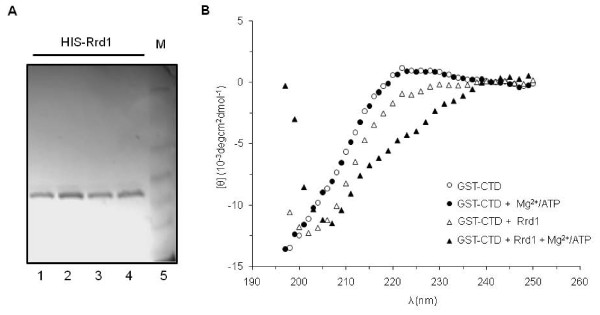
Figure 5.
Purified recombinant Rrd1 alters the structure of purified GST-CTD in vitro. A) Silver stained gel of purified recombinant HIS-Rrd1 from E. coli expression system (see Methods). Lanes 1-2 and 3-4 are elution samples from two independent purifications obtained directly from Talon affinity column; lane 5, molecular weight standard. B) Equimolar amounts (4.5 μM) of purified GST-CTD derived from the rrd1Δ mutant and the purified recombinant HIS-Rrd1 (triangle) were incubated at 30°C in phosphate buffer in the absence (opened symbol) and presence (closed symbol) of Mg2+/ATP. The resulting GST-CTD was re-purified free of the recombinant HIS-Rrd1 and subjected to CD analysis as in Figure 3. The result is the average of two independent experiments.