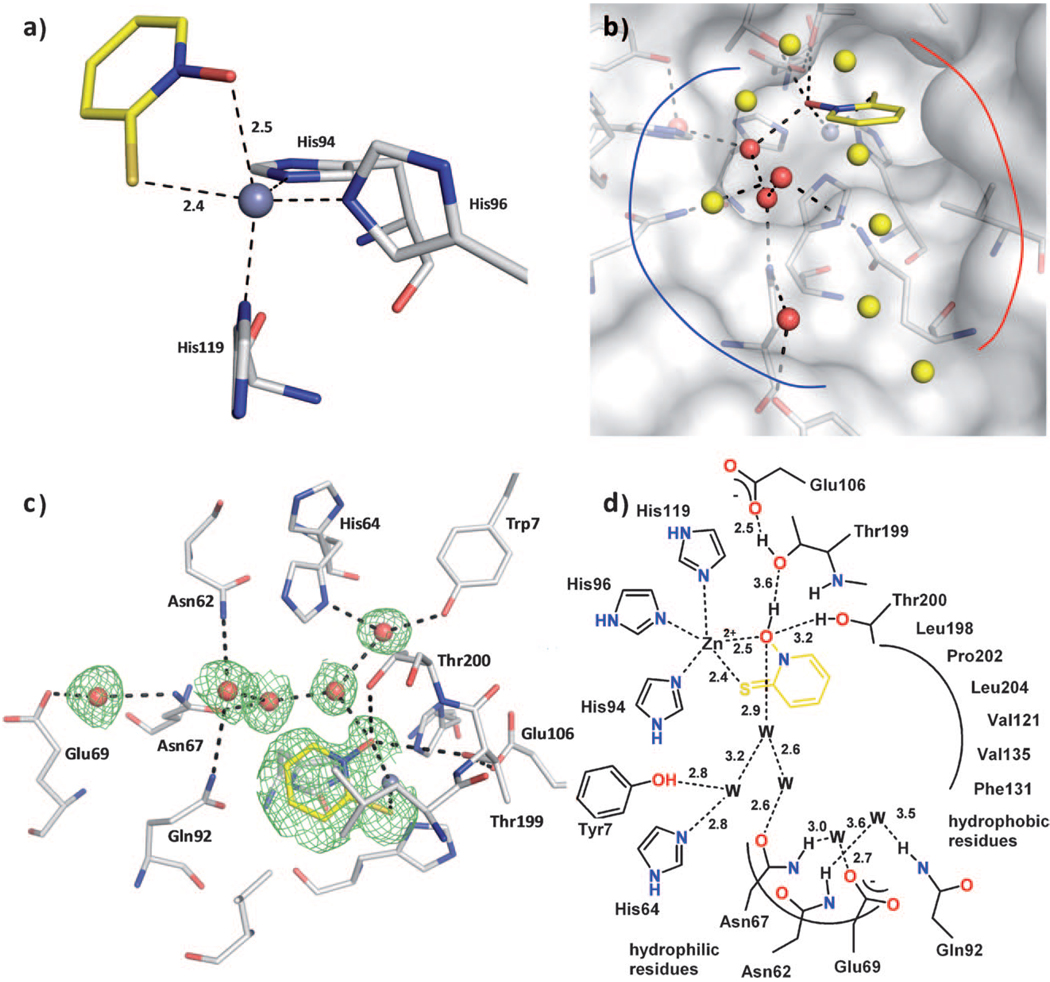
Figure 2.
Compound 4 binding to CA II. The protein residues are shown in stick representation (protein:  ; ligand:
; ligand:  ), and water molecules are shown as red and yellow spheres. The solvent accessible surface is represented in white in panel b). a) Coordination geometry of the active site Zn2+ ion upon binding of 4. The tetrahedral geometry is changed to distorted trigonal-bipyramidal arrangement with Nε2-His94 (2.0 Å), Nε2-His96 (2.0 Å), and the sulfur atom of 4 (2.4 Å) in the equatorial plane, whereas Nδ1-His119 (2.1 Å) and the oxygen atom of 4 (2.5 Å) are in the axial positions. The angles in the plane are 143.5° (S-Zn-His96), 109.5° (S-Zn-His94), and 101.7° (His94-Zn-His96). The two axial ligands and the Zn2+ ion form an angle of 161.2°. b) The binding pocket of CA II can be divided into a hydrophobic (red line) and a hydrophilic binding region (blue line). The fragment is stabilized by a hydrogen bond network which is mediated by five water molecules (red spheres) addressing the typical hydrophilic residues in CA II. The remaining waters in the binding pocket (yellow spheres) are connected to the hydrogen bond network as well, however not performing any further strong interactions to the protein. c) Detailed binding mode. Hydrogen bond interactions are indicated by the dashed lines. The equivalent distances are shown in the schematic representation of the binding mode in panel d). The difference electron density (Fo—Fc) for the ligands and directly interacting water molecules is shown at a σ level of 2.0. Atomic distances are shown in Å. The fragment coordinates with its sulfur and oxygen atom to the active site Zn2+ ion. Hydrogen bonds to Thr200 and Thr199 as well as a water network, which is spread over the entire binding pocket, linking the fragment to the common hydrophilic residues can be observed. d) Binding mode in schematic representation.
), and water molecules are shown as red and yellow spheres. The solvent accessible surface is represented in white in panel b). a) Coordination geometry of the active site Zn2+ ion upon binding of 4. The tetrahedral geometry is changed to distorted trigonal-bipyramidal arrangement with Nε2-His94 (2.0 Å), Nε2-His96 (2.0 Å), and the sulfur atom of 4 (2.4 Å) in the equatorial plane, whereas Nδ1-His119 (2.1 Å) and the oxygen atom of 4 (2.5 Å) are in the axial positions. The angles in the plane are 143.5° (S-Zn-His96), 109.5° (S-Zn-His94), and 101.7° (His94-Zn-His96). The two axial ligands and the Zn2+ ion form an angle of 161.2°. b) The binding pocket of CA II can be divided into a hydrophobic (red line) and a hydrophilic binding region (blue line). The fragment is stabilized by a hydrogen bond network which is mediated by five water molecules (red spheres) addressing the typical hydrophilic residues in CA II. The remaining waters in the binding pocket (yellow spheres) are connected to the hydrogen bond network as well, however not performing any further strong interactions to the protein. c) Detailed binding mode. Hydrogen bond interactions are indicated by the dashed lines. The equivalent distances are shown in the schematic representation of the binding mode in panel d). The difference electron density (Fo—Fc) for the ligands and directly interacting water molecules is shown at a σ level of 2.0. Atomic distances are shown in Å. The fragment coordinates with its sulfur and oxygen atom to the active site Zn2+ ion. Hydrogen bonds to Thr200 and Thr199 as well as a water network, which is spread over the entire binding pocket, linking the fragment to the common hydrophilic residues can be observed. d) Binding mode in schematic representation.