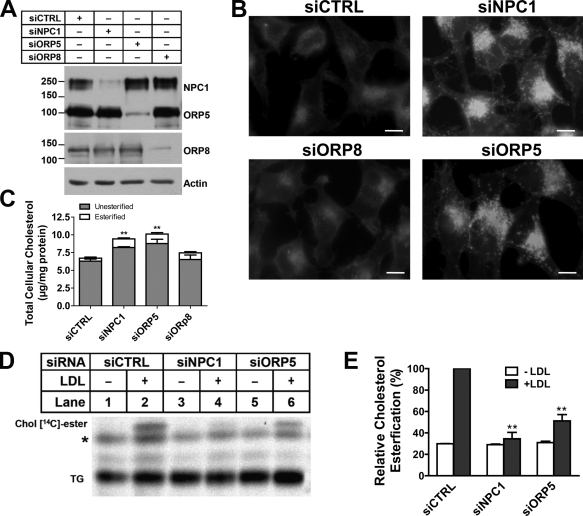
Figure 3.
ORP5 knockdown causes endosomal cholesterol accumulation and inhibits LDL-stimulated cholesterol esterification. (A) HeLa cells grown in medium A were transfected with siNPC1, siORP5, siORP8, or a universal control siCTRL for 48 h, followed by transfection with pcDNA4-ORP5 or pmCherry-ORP8 for 24 h. Efficiency of knockdown was analyzed by immunoblotting using polyclonal anti-NPC1, anti-ORP5, or anti-DsRed antibodies. The molecular mass of the proteins is indicated in kilodaltons. (B) HeLa cells grown in medium A were transfected with the indicated siRNAs for 54 h. Cells then received medium D supplemented with 50 µg/ml LDL for 18 h followed by processing for filipin staining. Fluorescent images are representative of four independent experiments with similar results. Bars, 10 µm. (C) Cells that received the same treatments as in B were lysed by 0.1 M NaOH and processed for measuring cellular free cholesterol as described in Materials and methods. Values were normalized to total cell proteins and are relative to siCTRL-transfected cells. The results are expressed as means + SD (error bars). **, P < 0.01. (D) HeLa cells grown in medium A were transfected with the indicated siRNAs for 48 h, followed by incubation in medium D for 18 h. Cells were then chased with LDL (50 µg/ml) and [14C]-palmitate in medium D for 6 h. Lipids extracted from cell lysates were standardized for total cell proteins and separated by TLC. Cholesteryl [14C]-esters were revealed by phosphorimaging. A representative phosphorimage of three experiments with similar results is shown. TG, triacylglycerols; *, unknown nonspecific lipids. (E) Quantification of cholesteryl [14C]-esters formed in D by densitometry. Values of siCTRL + LDL were arbitrarily set as 100, against which experimental data were normalized. The results are expressed as means + SD (error bars) and are representative of three independent experiments. Significant differences were show between siCTRL and siNPC1 or siORP5 in the presence of LDL. **, P < 0.01.