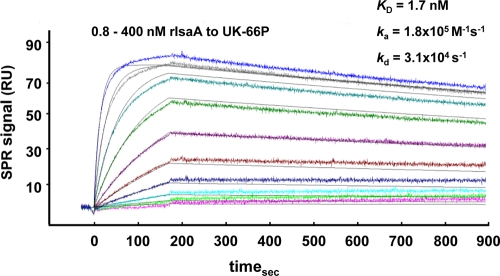
FIG. 1.
Quantitative analysis of the interaction of UK-66P with rIsaA, carried out using surface plasmon resonance (SPR). Various concentrations of rIsaA (0.8 to 400 nM) were flushed over the antibody UK-66P, immobilized on the sensor chip surface. Sensorgrams were recorded at a flow rate of 30 μl/min at 25°C. From these sensorgrams, an equilibrium dissociation constant (KD) of 1.7 nM was determined. Rate constants for association (ka) and dissociation (kd) were determined to be 1.8 × 105 M−1 s−1 and 3.1 × 10−4 s−1, respectively. The figure shows one representative result from two independent experiments yielding identical kinetic constants.