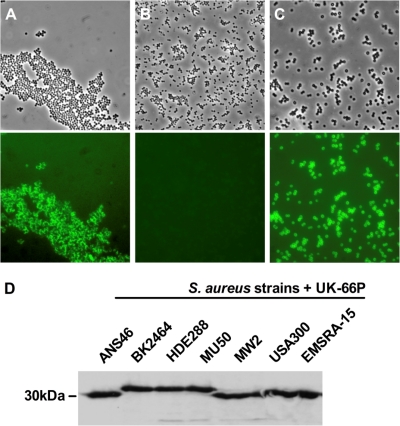
FIG. 2.
Display of UK-66P on the staphylococcal surface and positive binding to representative clinical S. aureus isolates are shown. Binding of FITC-labeled anti-mouse IgG to UK-66P was analyzed by conventional microscopy of S. aureus (top) and corresponding fluorescence microscopy with the data superimposed (bottom). (A) UK-66P binds specifically to wild-type S. aureus MA12. (B) The isogenic mutant strain MA12 ΔisaA failed to bind UK-66P. (C) The S. aureus protein A knockout strain (Cowan I Δspa) binds UK-66P, indicating no antibody cross-reactivity with protein A. Magnification, ×100. (D) Reactivity of UK-66P to IsaA of representative clinical isolates strain ANS46 (SCCmec III), strain BK2464 (SCCmec II), strain HDE288 (SCCmec IV), strain MU50 (vancomycin-resistant S. aureus [VRSA]), strain MW2 (CA-MRSA), strain USA300 (CA-MRSA), and strain EMSRA-15 (epidemic MRSA) was constant, as verified by Western blotting.