Abstract
The G-protein-coupled receptor CXCR4 acts as a coreceptor for human immunodeficiency virus type 1 (HIV-1) infection, as well as being involved in signaling cell migration and proliferation. Compounds that block CXCR4 interactions have potential uses as HIV entry inhibitors to complement drugs such as maraviroc that block the alternate coreceptor CCR5 or in cancer therapy. The peptide T140, which contains five arginine residues, is the most potent antagonist of CXCR4 developed to date. In a search for nonpeptide CXCR4 ligands that could inhibit HIV entry, three series of compounds were synthesized from 12 linear and branched polyamines with 2, 3, 4, 6, or 8 amino groups, which were substituted to produce the corresponding guanidines, biguanides, or phenylguanides. The resulting compounds were tested for their ability to compete with T140 for binding to the human CXCR4 receptor expressed on mammalian cells. The most effective compounds bound CXCR4 with a 50% inhibitory concentration of 200 nM, and all of the compounds had very low cytotoxicity. Two series of compounds were then tested for their ability to inhibit the infection of TZM-bl cells with X4 and R5 strains of HIV-1. Spermine phenylguanide and spermidine phenylguanide inhibited infection by X4 strains, but not by R5 strains, at low micromolar concentrations. These results support further investigation and development of these compounds as HIV entry inhibitors.
New therapeutics with novel mechanisms of action are needed for treatment of human immunodeficiency virus type 1 (HIV-1) infections. Maraviroc, a small molecule antagonist of the CCR5 receptor, was the first drug of its type to be approved, and it has proven to be effective against “R5” HIV that uses the CCR5 coreceptor (9). However, most patients that have failed conventional therapies also harbor “X4” HIV that uses CXCR4 or dualtropic viruses that can use either CCR5 or CXCR4. The appearance of the X4 viruses is associated with progression to AIDS symptoms (4), and drug resistance is more often linked to CXCR4-utilizing than to CCR5-utilizing HIV (39). Therefore, there is also a need to develop effective drugs that block CXCR4. Thus far, none of the CXCR4 antagonists in development have shown promise in HIV clinical trials, although some are being pursued as cancer therapeutics because CXCR4 may be involved in metastasis. Some CXCR4 inhibitors, such as the bicyclam AMD3100 and the monocyclam AMD3465, bind to a site located in the transmembrane domain of the receptor (13, 15, 26, 27, 30, 40). Like the CCR5-specific small-molecule inhibitors, these compounds are believed to block viral entry and chemokine signaling by allosteric mechanism(s). In contrast to CCR5 inhibitors, however, many of the CXCR4-specific inhibitors contain multiple positive charges that can interact with additional acidic residues in the extracellular loops of CXCR4 that are not conserved in CCR5 (2, 10, 27, 28, 35).
T140 is a 14-residue peptide antagonist of CXCR4 that blocks the binding of X4 strains of HIV-1 in vitro and displaces the natural agonist ligand SDF-1α with nanomolar affinity but does not bind to other chemokine receptors, such as CCR5 (30-32). It is much smaller than SDF-1α (14 residues versus 65 residues) but is rapidly degraded by proteolysis in serum (30) and has not been pursued as an HIV or cancer therapeutic. Recently, smaller cyclic peptides based on the structure of T140 (25, 37), as well as nonpeptide analogs (36), have been reported. However, the latter have 50% inhibitory concentrations (IC50s) at least 2 orders of magnitude higher than that of T140. The structure of T140 contains five Arg and two Lys residues; the latter can be substituted with uncharged side chains without loss of activity (30). ALX40-4C (N-α-acetyl-nona-d-arginine amide acetate) is another peptide antagonist of CXCR4 (8). An early clinical trial showed that it was well tolerated (7) and provided an important proof of principle that CXCR4 activity can be safely inhibited in humans. KRH-1636 is a CXCR4 antagonist that exhibits strong activity against X4 strains of HIV, competitively displaces SDF-1 from the receptor, and can be absorbed from the duodena of rats (17). Like T140 and ALX40-4C, KRH-1636 contains an Arg residue. An analog of this compound (KRH-2731) was reported to have enhanced activity and was claimed to be orally bioavailable in rats (24), but neither its structure nor any clinical trial results have been published. AMD3100 (plerixafor) is a bicyclam with eight secondary and tertiary amine groups (5, 6). Clinical trials for HIV have been abandoned because this compound showed poor efficacy and cardiotoxicity and was not orally bioavailable (16). The structurally similar monocylam AMD3465 has higher affinity for CXCR4 and is potent against X4 HIV strains in vitro (IC50, 1 to 10 nM) (19) but is also not orally bioavailable (14). AMD070 (also known as AMD11070), which has two aromatic rings in addition to a primary and a tertiary amine, has an IC50 of 2 to 26 nM against an X4 HIV strain and is orally bioavailable (22, 29).
Previous studies at Novaflux and Drexel University have led to the identification and characterization of a new HIV-1 inhibitor, NB325 (20). This is a biguanide-based, low-molecular-weight oligomer (∼800 to 1,400 Da) that inhibits infection via specific interactions with CXCR4 and has been shown to be a potent HIV-1 inhibitor in a number of in vitro assays (3, 34). NB325 functions by specifically preventing the interaction of HIV-1 gp120 with the extracellular loop 2 (ECL2) of the CXCR4 coreceptor. Persistent interactions between NB325 and CXCR4 result in prolonged inhibition of HIV-1 infection (33). NB325 blocks SDF-1α signaling through CXCR4 but not SDF-1α attachment (34). The specificity of this new class of compounds as CXCR4 antagonists has also been confirmed by competitive binding with the peptide antagonist T140 (described below) and the inhibition of Ca2+ flux induced by the natural chemokine agonist SDF-1α (CXCL12; T. Sakmar, The Rockefeller University [unpublished data]). However, because NB325 is a polydisperse preparation consisting of a homologous series of compounds with 4 to 10 biguanide groups, its approval for clinical use is unlikely. We therefore developed a series of discrete, small-molecule inhibitors containing multiple guanide or biguanide groups to test their ability to inhibit HIV-1 binding to CXCR4. The compounds were initially screened for their ability to inhibit the binding and cross-linking of a fluorescently labeled photoactive analog of T140 to CXCR4. The most effective inhibitors of T140 binding and cross-linking were subsequently tested for their ability to inhibit HIV infection. Some of these compounds inhibited infection by X4, but not R5 or X4/R5, strains of HIV. The results demonstrate the potential of these compounds in the development of X4 HIV entry inhibitors, but these compounds will have to be improved upon substantially to match the inhibition of R5 HIV by maraviroc.
MATERIALS AND METHODS
Starting material amine compounds.
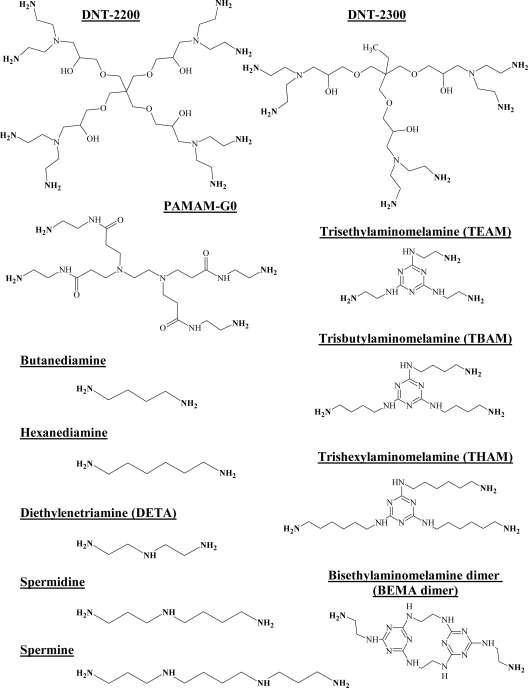
Putrescine dihydrochloride and spermine tetrahydrochloride were purchased from Sigma (St. Louis, MO). 1,4-diaminobutane, 1,6-hexanediamine dihydrochloride, 1,6-hexanediamine, diethylenetriamine, and spermidine were purchased from Acros (Morris Plains, NJ). StarburstPAMAM G(0.0) was purchased from Dendritech, Inc. (Midland, MI). Priostar dendrimers DNT-2200 and DNT-2300 were purchased from Dendritic Nanotechnologies, Inc. (Mt. Pleasant, MI).
(i) THAM synthesis.
Trishexylaminomelamine (THAM) was synthesized according to the method of Kaiser et al. (18). Cyanuric chloride (TCI America, Tokyo, Japan) was reacted with a 3.3 molar ratio of N-BOC-1,6-diaminohexane (Alfa Aesar, Ward Hill, MA) in refluxing water for 1.25 h. The pH of the solution was monitored with phenolphthalein, and the pink color was maintained by gradual addition of a 0.4 M sodium carbonate solution. The reaction was lyophilized and the BOC groups removed by treating the residue with 100% trifluoroacetic acid (TFA) for 2 h at room temperature. The deprotected product was purified by reversed-phase high-pressure liquid chromatography (HPLC).
(ii) TEAM synthesis.
The synthesis of trisethylaminomelamine (TEAM) was begun as described above using N-BOC-1,2-diaminoethane produced as previously described (23). The initial reaction gave the bis addition product (after deprotection) with one unreacted chlorine. Treatment of this compound with a concentrated ammonia solution in water resulted in the formation of a dimeric compound (BEMA dimer). Treatment of the bis addition product before deprotection with excess ethylenediamine, followed by deprotection, gave the desired trisethylamino product, which was purified by reversed-phase HPLC.
(iii) TBAM synthesis.
Synthesis of trisbutylaminomelamine (TBAM) was similar to process for the TEAM synthesis. The initial bis addition product, after deprotection, was treated with excess 1,4-butanediamine and refluxed for 2 h in water. Purification by HPLC under acidic conditions did not result in separation of the desired product from the excess diamine. However, under basic conditions the product bound and was not eluted. Subsequent washing with 0.1% TFA in water eluted the desired product without the contaminating diamine.
Synthesis of guanide derivatives.
The reaction of polyamine starting materials was carried out using 10% excess O-methylisourea sulfate salt (MP Biomedicals) in water at basic pH. Free amine compounds were added to the O-methylisourea in water and incubated at 37 to 42°C for 1 to 2 h. Reactions with acidified amine starting materials (HCl or TFA salts) were neutralized with triethylamine (TEA) before being allowed to react. Products were purified by reverse phase HPLC and characterized by matrix-assisted laser desorption ionization (MALDI) mass spectrometry.
Synthesis of biguanide derivatives.
S-Methyl-guanylisothiouronium iodide was prepared as previously described (11). Briefly, iodomethane (Acros) was added dropwise to a suspension of 2-imino-4-thiobiuret (Aldrich) in 100% ethanol while stirring at room temperature. The reaction flask was shaken at 37°C for 2 h. The solvent was removed by evaporation, and the residue was washed with cold diethyl ether to give a white, crystalline solid that was collected by filtration and used without further purification.
Addition of the biguanide reagent (S-methyl-guanylisothiouronium iodide, in excess) to the amine compounds was done in 100% ethanol as previously described (38) at room temperature or 60°C or in water at room temperature, 37°C, or 65°C. The pH of the reactions were monitored and adjusted with TEA or sodium carbonate when it fell below a pH of ∼8. Reaction times varied between 5 h and 5 days. Products were purified and characterized as described above.
Synthesis of phenylguanide derivatives.
S-Methyl-N-phenylisothiouronium iodide was prepared as described above by addition of methyl iodide to 1-phenyl-2-thiourea (Acros). Addition to the amine compounds was accomplished by refluxing 10% excess of the S-methylthiourea compound with the amine in 50% acetonitrile-water overnight. Reactions with acidified amine starting materials (HCl or TFA salts) were neutralized with TEA as described above. The products were purified and characterized as described above.
Synthesis of a photoactive, fluorescent T140 peptide.
A fluorescent, photoactive cross-linkable derivative of the CXCR4 binding peptide T140 (Ac-RRNalCYRBpaD-KPYRCitCR [Nal, naphthylalanine; Cit, citruline; Bpa, 4-benzoylphenylalanine]) (30, 32) was synthesized on a solid support using standard Fmoc (9-fluorenylmethoxy carbonyl) chemistry. Fmoc amino acids with appropriate side-chain protecting groups were purchased from Advanced Chemtech (Louisville, KY). After cleavage from the resin and side-chain deprotection, the crude peptide was purified by reversed-phase HPLC. A dilute solution of the peptide in 10 mM ammonium bicarbonate (pH 8) was stirred while bubbling air through the solution at 4°C until oxidation of the intrapeptide disulfide bond was complete as analyzed by MALDI mass spectrometry. The solution was lyophilized, and the oxidized peptide was purified by reversed-phase HPLC.
The purified peptide was fluorescently labeled on the ɛ-amine of the d-lysine residue by reaction with N-hydroxysuccinimide-activated 6-(fluorescein-5-(and-6)-carboxamido)hexanoic acid (Invitrogen, Carlsbad, CA) in dry dimethylformamide with 10% pyridine overnight at 4°C. The labeled peptide was purified by reversed-phase HPLC.
CXCR4-T140 cross-link inhibition assay.
The ability of the guanide, biguanide, and phenylguanide compounds to interact with CXCR4 was tested by a cross-link inhibition experiment. Serial dilutions of the inhibitor compounds were incubated with approximately 1.5 × 105 CXCR4-expressing cells (syn-CXCR4-C9 in Cf2Th) (1) for 30 min on ice. The photoactive, fluorescent T140 peptide was added to a final concentration of 75 nM, followed by incubation on ice for an additional 30 min. Cross-linking of the T140 peptide to the receptor was carried out by irradiation at 365 nm in a Rayonet Photochemical Reaction chamber (Southern New England UV Company, Hamden, CT) four times for 5 min with 5 min of cooling on ice between irradiations. The cell pellets were spun down, washed with 1× phosphate-buffered saline (PBS), resuspended in PAGE sample buffer with 40 mM dithiothreitol, and run on an SDS-PAGE gel. The gels were imaged on a Bio-Rad Molecular Imager FX (Hercules, CA), and the fluorescent CXCR4 bands were quantified with Bio-Rad Quantity One software.
CCR5-maraviroc analog cross-link inhibition assay.
A maraviroc analog containing a benzophenone cross-linking group and a fluorescein tag were prepared (synthesis to be published later). This compound was used to test the guanide, biguanide, and phenylguanide compounds for interaction with CCR5 in an assay similar to that used for the CXCR4-T140 inhibition assay above.
Assay for anti-HIV activity.
Cell-free virus supernatants were prepared in PHA blast cultures as described elsewhere (21). TZM-bl cells (41) were obtained from the NIH AIDS Research and Reference Reagent Program; 4 × 104 cells were plated per well. CXCR4 inhibitors were added to the cells for 1 h at 37°C. Predetermined dilutions of the virus supernatants in 15 μg of DEAE-dextran (Sigma)/ml were then added to the wells containing the cells plus inhibitor. Three days later, the cells were lysed, and the luciferase activity was measured (Bright-Glo Luciferase assay; Promega, Madison WI).
Toxicity assays.
Compound cytotoxicity was evaluated after 48 h of exposure using a CellTiter 96 aqueous nonradioactive cell proliferation assay (MTS) according to the manufacturer's instructions (Promega, Madison, WI) with the human breast cancer cell line MDA-MB-231 or the TZM-bl cell line used in the HIV infectivity assay.
RESULTS
Guanide, biguanide, and phenylguanide synthesis.
The results of the compound syntheses are summarized in Table 1. Starting material compounds (see Fig. 1 for structures) are listed in the first column with the number of amines available for addition of guanide, biguanide, or phenylguanide groups (see Fig. 2) in parentheses. The results for each column show the number of amine groups derivatized. In data presented with “or” in Table 1, two fractions were isolated with each of the respective products (e.g., both the monophenylguanide and the bisphenylguanide additions to butanediamine were isolated and tested separately). In data presented with a dash in Table 1, the designated products were inseparable by HPLC (e.g., DNT2300 yielded a mixture of compounds with either five or six guanide groups per molecule).
TABLE 1.
Compounds synthesized
| Starting amine (n)a | No. of amines derivatizedb |
||
|---|---|---|---|
| Guanide | Biguanide | Phenylguanide | |
| Butanediamine (2) | 2 | 2 | 1 or 2 |
| Hexanediamine (2) | 2* | 1 or 2 | 1 or 2 |
| Diethylenetriamine (3) (DETA) | 2 | 3† | 3‡ |
| Spermidine (3) | 2 or 2-3 | 1-2 | 2 or 3 |
| Spermine (4) | 2 | 2 | 3-4 |
| Trisethylaminomelamine (3) (TEAM) | 3 | 1 | 3 |
| Trisbutylaminomelamine (3) (TBAM) | 3 | 1-2 | 2 or 3 |
| Trishexylaminomelamine (3) (THAM) | 3 | 1-3 | 2 |
| Bisethylaminomelamine dimer (2) (BEMA dimer) | 2 | ND | 1-2 |
| PAMAM G0 (4) | 4* | 3-4 | 4** |
| DNT2300 (6) | 5-6* | 3-6¶ | 6 |
| DNT2200 (8) | 8 | 1-5 | 8 |
n, number of reactive amines in each starting material.
*, +15 mass also (O-Me replace NH2); **, small amount of +5 phenylguanide groups; †, cyanoamines and biguanides; ‡, cyclic by-products; ¶, masses 4 amu too low. ND, synthesis not done.
FIG. 1.
Synthetic starting materials. The structures of the starting material polyamines used to synthesize guanide, biguanide, and phenylguanide derivatives are shown. Reactive amines are noted in boldface.
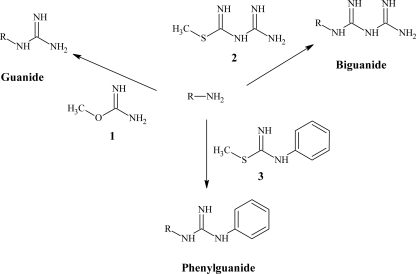
FIG. 2.
Generic synthetic reaction scheme. Primary and secondary amines treated with the reactive compounds O-methylisourea (compound 1), S-methyl-N-guanylisothiourea (compound 2), or S-methyl-N-phenylisothiourea (compound 3) give the desired guanide, biguanide, or phenylguanide derivatives.
Other deviations from the expected products are noted in Table 1. In three of the guanide addition reactions, additional +15- and sometimes +30-atomic mass unit (amu) products were copurified with the isolated products. For these compounds, 20 to 50% of the isolated product was observed to be the +15 product, with a small amount (<10%) of the +30 product. These compounds were tested for activity as isolated; later, it was discovered that these products were the result of loss of ammonia during the substitution reaction instead of the expected loss of methanol. Treatment of these compounds with concentrated ammonium hydroxide can replace the O-methyl groups and yield the desired products (see Fig. S1 in the supplemental material), but the effect of this reaction on the activity of the compound was not tested.
The reaction of diethylenetriamine (DETA) with the phenylguanide reagent resulted in some cyclic by-products (see Fig. S2 in the supplemental material) that can be encountered when the reactive amine groups are closely spaced. These same mechanisms are probably involved in the formation of the products labeled “cyanoamine and biguanides” in the reaction of DETA with S-methyl-guanylisothiourea. This reaction proceeds through the loss of urea and the formation of a cyclic product, as seen with the phenylguanides, or to the formation of a cyanoamine. However, mass spectrometry is unable to differentiate between the two by-products. Finally, the addition of biguanides to the dendrimer DNT2300 resulted in an unexplained deficit of 4 amu from the isolated products regardless of the number of biguanide groups added.
CXCR4-T140 cross-link inhibition assay.
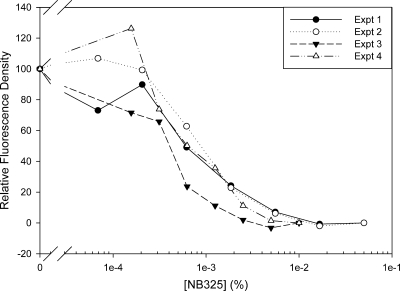
The compounds were tested for CXCR4 binding by inhibiting the cross-linking of a photoactive, fluorescent derivative of the known CXCR4-binding peptide T140. Initially, this assay was tested by using NB325 as the inhibitor. Figure 3 shows the results for four separate experiments. Although the relative fluorescence density (signal strength) varies from gel to gel, the IC50s are quite reproducible, varying between 0.0006 and 0.001%. Assuming a median molecular weight of 800 to 1,400 for NB325 gives an IC50 of ∼10 μM.
FIG. 3.
Inhibition of T140-fluorescein cross-linking to CXCR4. CXCR4-expressing cells were incubated with serial dilutions of the polybiguanide NB325 and then allowed to bind the fluorescent, photoactive T140 derivative. After irradiation at 365 nm, the samples were dissolved in SDS, run on an SDS-PAGE gel, and imaged with a fluorescent gel imager. Inhibition curves for four separate experiments with one reading per data point are shown.
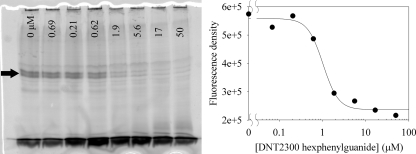
A representative gel and analysis are shown in Fig. 4 for the dendrimer derivative DNT2200 octaguanide. The amount of fluorescence was quantified as the fluorescence density of a box encompassing the doublet band (CXCR4) in the middle of the gel.
FIG. 4.
Representative inhibition experiment gel and graphical analysis. Serial dilutions of the inhibitor (DNT2300 hexaphenylguanide) were incubated with CXCR4-expressing cells. The fluorescent, photoactive derivative of T140 was added, followed by irradiation at 365 nm to induce cross-linking between the peptide and receptor. Samples were dissolved in SDS, run on an SDS-PAGE gel, and imaged with a fluorescent gel reader. The concentration of inhibitor is marked at the top of each lane. The intensities of the fluorescent CXCR4 bands (doublet indicated by arrow) were quantified and plotted with Sigma Plot to determine an approximate IC50 (∼1 μM). A Western blot of the gel incubated with monoclonal antibody 1D4 was used to confirm the identity of the CXCR4 bands and their uniformity across the gel (not shown).
The results of the CXCR4-T140 cross-link inhibition assay are shown in Table 2. The IC50s are given for the starting material amines and the respective guanide, biguanide, and phenylguanide derivatives. Most of the starting amines had IC50s greater than or equal to the highest concentration tested (200 μM), with the exception of spermine, which inhibited the T140 cross-linking with an IC50 of 75 μM.
TABLE 2.
CXCR4/T-140-Fl inhibition data
| Starting amine | IC50 (μM)a |
|||
|---|---|---|---|---|
| Starting amine | Guanide | Biguanide | Phenylguanide | |
| Butanediamine | >200 | 50 | >200 | >200 |
| Hexanediamine | ≥200 | >200 | >200 | 150 (1), 20 (2) |
| Diethylenetriamine (DETA) | ≥200 | 50 | >200 | 15 (1), 6 (2) |
| Spermidine | >200 | 8 (2), 10 (3) | 65 (1-2) | 0.2 (2), 0.2 (3) |
| Spermine | 75 | 10 (2) | 4-8 (2) | 0.5 (3-4) |
| Trisethylaminomelamine (TEAM) | >200 | 20 | >200 (1) | 1.5 (3) |
| Trisbutylaminomelamine (TBAM) | ≥100 | 10 | >200 (1-2) | 6 (2), 20 (3) |
| Trishexylaminomelamine (THAM) | >200 | 2 | 45 (1-3) | >200 (2) |
| BEMA dimer | >200 | 50 | ND | 15 (1), 70 (2) |
| PAMAM GO | >200 | 4 | >50 (3-4) | 3 |
| DNT2300 | >200 | 8 | 7.5 (3-6) | 1.5 |
| DNT2200 | ≥200 | 15 | ≥200 (1-5) | 3.5 |
For comparison, NB325, assuming an average molecular weight of ∼1,000, has an IC50 of 10 μM. Numbers in parentheses indicate the number of groups added. ND, synthesis not done.
As a general rule, the inhibition activity increased in the following order: amines < biguanides < guanides < phenylguanides. However, there are a couple of notable exceptions. The butanediamine was only active as the guanide derivative, with an IC50 of 50 μM. For THAM, the guanide and biguanide derivatives were active, but interestingly, the phenylguanide compound showed no inhibition. The derivatives of linear polyamines (butanediamine, hexanediamine, diethylenetriamine, spermidine, and spermine) generally increased in activity as they increased in size. As a group, the spermidine and spermine derivatives were the most active, and the most inhibition was exhibited by spermidine bisphenylguanide and spermidine trisphenylguanide, with IC50s of 200 nM.
The melamine core guanide derivatives (TEAM, TBAM, and THAM) increased in activity as the chain length increased (ethyl < buty < hexyl). Conversely, the corresponding phenylguanides showed the opposite trend, with the THAM bisphenylguanide actually showing no inhibition at the highest concentration tested (200 mM). The dendrimer derivatives (PAMAM-G0, DNT2300, and DNT2200) were generally equally active as the number of reactive amines varied from 4 to 6 to 8.
Inhibition of HIV infection.
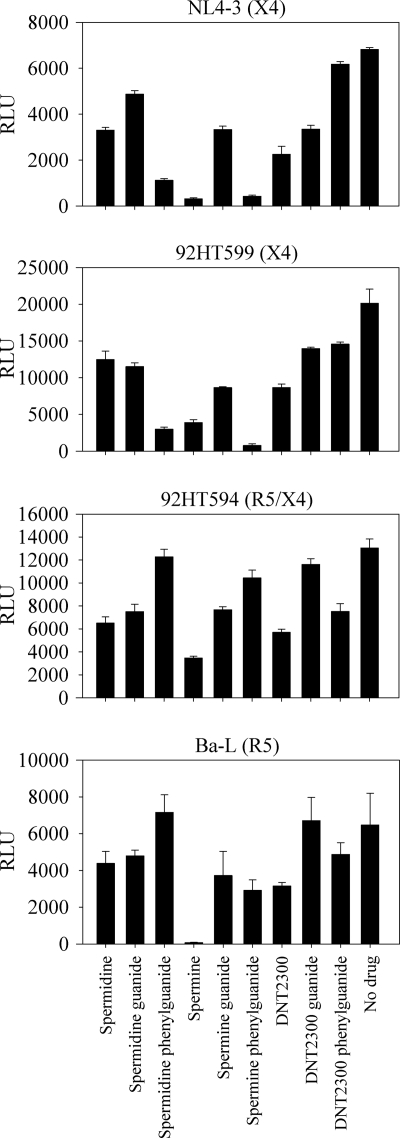
Six of the most active compounds, and the amines from which they were derived, were selected for preliminary testing as HIV infection inhibitors. Spermidine, spermine, DNT2300, and their guanide and phenylguanide derivatives were tested for their ability to inhibit HIV infection at 10 μM in the TZM-bl assay. The compounds were screened against CXCR4-, CCR5-, and R5/X4-tropic virus strains. The spermidine and spermine phenylguanides were effective against both X4 strains, with no inhibition of the R5/X4 strain and limited inhibition of the R5 strain (Fig. 5).
FIG. 5.
Initial anti-HIV activity screen. The series (amine, guanide, and phenylguanide) of the most active derivatives (spermidine, spermine, and DNT2300) in the T140 cross-link inhibition assay were screened for anti-HIV activity. TZM-bl cells were plated and preincubated with the inhibitors at a final concentration of 10 μM. Previously titered viral supernatants were added, and the cells were incubated for 3 days. Cells were lysed, and the TAT-driven reporter luciferase activity was measured using a Bright-Glo luciferase assay. Experiments were performed in triplicate with errors shown as means ± the standard error of the mean (SEM). RLU, relative luminescence units.
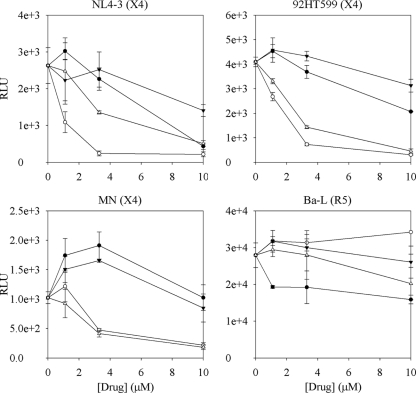
The underivatized amine spermine appeared to be active against both R5 and X4 strains at this concentration. However, in a second TZM-bl assay run to investigate the dose-response of the active phenylguanides and their respective amine starting materials, the phenylguanides were active at ∼10-fold-lower concentrations than the corresponding amines against three X4 strains (Fig. 6 and 7A). None of the compounds showed significant activity (>2-fold reduction) against an R5 virus. At 10 μM, spermine did not significantly inhibit fluorescent T140 binding to CXCR4 (see above) or the binding of a fluorescent maraviroc analog to CCR5, nor was it cytotoxic to TZM-bl cells (data not shown). Therefore, the reason for the observed inhibition of X4 and R5 infection by the underivatized amines is unclear.
FIG. 6.
HIV inhibition dose-response trials. Spermine (•), spermine phenylguanide (○), spermidine (▾), and spermidine phenylguanide (▵) were tested for dose-response activity in the TZM-bl luciferase anti-HIV assay. Increased activity against CXCR4-using viral strains were observed with the phenylguanides compared to the underivatized parent amines. Experiments were performed in triplicate with errors shown as means ± the SEM. RLU, relative luminescence units.
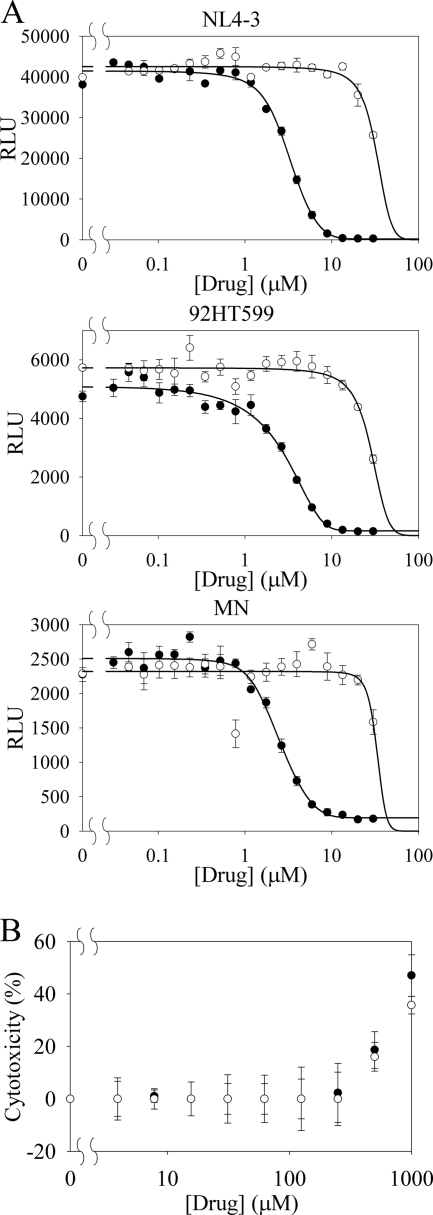
FIG. 7.
Spermidine phenylguanide activity and cytotoxicity. (A) HIV inhibition activity of spermidine phenylguanide (•) and spermidine (○) against three CXCR4 specific HIV clones (NL4-3, 92HT599, and MN) using the TZM-bl assay. Experiments were performed in triplicate with errors shown as means ± the SEM. RLU, relative luminescence units. (B) An MTS assay was used to measure the cytotoxicity (% of control TZM-bl cells) for spermidine phenylguanide (•) and spermidine (○) against the TZM-bl cells. Experiments were performed in duplicate with errors shown as means ± the SEM.
Cytotoxicity.
The toxicity of the compounds was evaluated by using an MTS dye reduction assay in a CXCR4 expressing human breast cancer cell line for all of the compounds (see Table S1 in the supplemental material) and in TZM-bl cells for spermidine phenylguanide and the spermidine control (Fig. 7B). The majority of the compounds showed no toxicity at the highest concentrations tested against the MDA-MB-231 cells. Spermidine and spermidine phenylguanide both had CC50 values of ∼1,000 μM against the TZM-bl cells while showing HIV inhibition at <5 μM (Fig. 7A).
DISCUSSION
We have synthesized a novel series of compounds containing multiple guanide, biguanide, or phenylguanide groups on linear aliphatic or dendrimer scaffolds. These compounds bear some resemblance to the polyethylenehexamethylene biguanide NB325, as well as to the CXCR4 antagonist peptide T140, which has five guanide groups on the side chains of arginine residues. Among the new compounds, the bis- and trisphenylguanide derivatives of spermidine exhibited the highest affinity for CXCR4, with an IC50 of 200 nM in competition assays with T140. This is a 50-fold-higher affinity than found for NB325 in this assay and is comparable to that of other nonpeptide analogs of T140 that have been reported (36), although it does not approach the nanomolar affinity of T140 itself.
The best T140 inhibitors were also tested for their ability to inhibit HIV infection. The spermine and spermidine derivatives and amines were tested against a panel of X4, R5, or X4/R5 isolates. Spermine phenylguanide and spermidine phenylguanide were effective at inhibiting the infection of TZM-bl cells by X4, but not R5, HIV strains, a finding consistent with the observation that they bound to CXCR4 but not CCR5. In fact, none of the compounds in Table 2 inhibited the binding of a fluorescent maraviroc analog to CCR5 (data not shown). Spermidine phenylguanide had an IC50 of 3 μM when tested for inhibition of three X4 strains (NL4-3, 92HT599, and MN). The HIV inhibition data were also consistent with the T140 inhibition results in that the phenylguanides were more active than the related guanide or biguanide compounds. However, the underivatized parent amines appeared to show inhibition against both X4 and R5 strains of HIV, even though they were not effective in inhibiting T140 binding and cross-linking to CXCR4, suggesting that they were acting via another mechanism.
In experiments with primary human CD4+ T lymphocytes as the target cells, NB325 inhibited HIV-1 IIIB infection with IC50 concentrations of ca. 0.01% (∼71 μM) (34). This is ∼50-fold higher than the concentrations of spermine phenylguanide and spermidine phenylguanide required to inhibit X4 HIV infection, which is consistent with the higher affinity for CXCR4 relative to NB325. Since NB325 is a mixture, it is of course possible that it contains a component with greater activity. We attempted to fractionate NB325 by reversed-phase, ion exchange, and size exclusion chromatography but were unable to isolate a fraction with higher affinity for CXCR4 (data not shown).
The optimal structure for a nonpeptide CXCR4 antagonist cannot be readily predicted from the data available. Attempts to develop small cyclic peptide and nonpeptide analogs from T140 (12, 36, 37) have pointed to the importance of both a guanide functional group (in the form of an arginine side chain) and an aromatic group (napthyl). This is consistent with our observation that the phenylguanides were more active than either the guanides or the biguanides and suggests that using a larger aromatic moiety such as naphthalene may increase the observed CXCR4 binding compared to our initial phenylguanide derivatives.
The positioning of the aromatic group also appears to be important. The most active phenylguanides (spermidine and spermine derivatives), with the aromatic groups on the ends and sides of the main chain of the molecule, have at least an order of magnitude better binding than the best melamine derivative (THAM trisguanide), which has the aromatic group in the center of the molecule with the charges surrounding it. There also appears to be a necessary balance between charge and hydrophobic character. This is exemplified in the series of melamine-derived phenylguanides in which increasing the length of the spacers between the melamine and phenylguanide groups, which increases overall hydrophobicity, led to a progressive loss of activity.
The spacing between the positively charged groups, at least in the linear compounds, also appears to be important. Experiments in the development of NB325 indicated that optimal efficacy was found when an alternating 2:6 (ethyl-, hexyl-) spacing between biguanides was present compared to evenly spaced ethyl-, butyl-, or hexyl-based polybiguanides (20). In the experiments reported here, the most active core molecules (spermine and spermidine) have spacings of three or four carbons between the reactive amine groups, whereas the less active diethylenetriamine and hexanediamine cores have two and six carbons, respectively. However, the flexibility of the polyamine backbones versus those of the polybiguanides makes comparison of the distances between the charged groups in the two types of molecules difficult.
The compounds reported here, especially the phenylguanides, have significant advantages over the polybiguanides in that they exhibit higher affinity for CXCR4 and are discrete compounds rather than heterogeneous mixtures; therefore, they represent more promising leads for developing nonpeptide inhibitors of X4 HIV infection. Their very low cytotoxicity makes them promising candidates for future animal and clinical studies and their nonpeptide structures may give them greater serum stability than T140.
Supplementary Material
Acknowledgments
This study was supported by Public Health Service grant AI076965 (M.L.) from the National Institute of Allergy and Infectious Diseases (NIAID). S.K.W. was supported by a fellowship from grant P20 RR-16455 from the National Center for Research Resources. The synthesis of the T140 and maraviroc analogs was supported by grant AI064107 from NIAID to Edward A. Dratz.
We thank Tarin Richards for performing CCR5 inhibition assays, Junwei Shen and Paul Grieco for the maraviroc analog, and Edward A. Dratz for the use of his peptide synthesizer. We thank Mary Cloninger for a gift of PAMAM G0. Cf2Th-CXCR4 cells from Joseph Sodroski were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, National Institutes of Health.
Footnotes
Published ahead of print on 11 October 2010.
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1.Babcock, G. J., T. Mirzabekov, W. Wojtowicz, and J. Sodroski. 2001. Ligand binding characteristics of CXCR4 incorporated into paramagnetic proteoliposomes. J. Biol. Chem. 276:38433-38440. [DOI] [PubMed] [Google Scholar]
- 2.Billick, E., C. Seibert, P. Pugach, T. Ketas, A. Trkola, M. J. Endres, N. J. Murgolo, E. Coates, G. R. Reyes, B. M. Baroudy, T. P. Sakmar, J. P. Moore, and S. E. Kuhmann. 2004. The differential sensitivity of human and rhesus macaque CCR5 to small-molecule inhibitors of human immunodeficiency virus type 1 entry is explained by a single amino acid difference and suggests a mechanism of action for these inhibitors. J. Virol. 78:4134-4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Catalone, B. J., T. M. Kish-Catalone, L. R. Budgeon, E. B. Neely, M. Ferguson, F. C. Krebs, M. K. Howett, M. Labib, R. Rando, and B. Wigdahl. 2004. Mouse model of cervicovaginal toxicity and inflammation for preclinical evaluation of topical vaginal microbicides. Antimicrob. Agents Chemother. 48:1837-1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Connor, R. I., K. E. Sheridan, D. Ceradini, S. Choe, and N. R. Landau. 1997. Change in coreceptor use correlates with disease progression in HIV-1-infected individuals. J. Exp. Med. 185:621-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Clercq, E. 2009. The AMD3100 story: the path to the discovery of a stem cell mobilizer (Mozobil). Biochem. Pharmacol. 77:1655-1664. [DOI] [PubMed] [Google Scholar]
- 6.Donzella, G. A., D. Schols, S. W. Lin, J. A. Este, K. A. Nagashima, P. J. Maddon, G. P. Allaway, T. P. Sakmar, G. Henson, E. De Clercq, and J. P. Moore. 1998. AMD3100, a small molecule inhibitor of HIV-1 entry via the CXCR4 coreceptor. Nat. Med. 4:72-77. [DOI] [PubMed] [Google Scholar]
- 7.Doranz, B. J., L. G. Filion, F. Diaz-Mitoma, D. S. Sitar, J. Sahai, F. Baribaud, M. J. Orsini, J. L. Benovic, W. Cameron, and R. W. Doms. 2001. Safe use of the CXCR4 inhibitor ALX40-4C in humans. AIDS Res. Hum. Retrovir. 17:475-486. [DOI] [PubMed] [Google Scholar]
- 8.Doranz, B. J., K. Grovit-Ferbas, M. P. Sharron, S. H. Mao, M. B. Goetz, E. S. Daar, R. W. Doms, and W. A. O'Brien. 1997. A small-molecule inhibitor directed against the chemokine receptor CXCR4 prevents its use as an HIV-1 coreceptor. J. Exp. Med. 186:1395-1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dorr, P., M. Westby, S. Dobbs, P. Griffin, B. Irvine, M. Macartney, J. Mori, G. Rickett, C. Smith-Burchnell, C. Napier, R. Webster, D. Armour, D. Price, B. Stammen, A. Wood, and M. Perros. 2005. Maraviroc (UK-427,857), a potent, orally bioavailable, and selective small-molecule inhibitor of chemokine receptor CCR5 with broad-spectrum anti-human immunodeficiency virus type 1 activity. Antimicrob. Agents Chemother. 49:4721-4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dragic, T., A. Trkola, D. A. Thompson, E. G. Cormier, F. A. Kajumo, E. Maxwell, S. W. Lin, W. Ying, S. O. Smith, T. P. Sakmar, and J. P. Moore. 2000. A binding pocket for a small molecule inhibitor of HIV-1 entry within the transmembrane helices of CCR5. Proc. Natl. Acad. Sci. U. S. A. 97:5639-5644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eilingsfeld, H., and H. Scheuermann. 1967. Synthese Von 1.3.5-Triazinen. Chemische Berichte-Recueil. 100:1874-1891. [Google Scholar]
- 12.Fujii, N., S. Oishi, K. Hiramatsu, T. Araki, S. Ueda, H. Tamamura, A. Otaka, S. Kusano, S. Terakubo, H. Nakashima, J. Broach, J. Trent, Z. Wang, and S. Peiper. 2003. Molecular-size reduction of a potent CXCR4-chemokine antagonist using orthogonal combination of conformation- and sequence-based libraries. Angew. Chem. Int. 42:3251-3253. [DOI] [PubMed] [Google Scholar]
- 13.Gerlach, L. O., R. T. Skerlj, G. J. Bridger, and T. W. Schwartz. 2001. Molecular interactions of cyclam and bicyclam non-peptide antagonists with the CXCR4 chemokine receptor. J. Biol. Chem. 276:14153-14160. [DOI] [PubMed] [Google Scholar]
- 14.Hatse, S., K. Princen, E. De Clercq, M. M. Rosenkilde, T. W. Schwartz, P. E. Hernandez-Abad, R. T. Skerlj, G. J. Bridger, and D. Schols. 2005. AMD3465, a monomacrocyclic CXCR4 antagonist and potent HIV entry inhibitor. Biochem. Pharmacol. 70:752-761. [DOI] [PubMed] [Google Scholar]
- 15.Hatse, S., K. Princen, L. O. Gerlach, G. Bridger, G. Henson, E. De Clercq, T. W. Schwartz, and D. Schols. 2001. Mutation of Asp(171) and Asp(262) of the chemokine receptor CXCR4 impairs its coreceptor function for human immunodeficiency virus-1 entry and abrogates the antagonistic activity of AMD3100. Mol. Pharmacol. 60:164-173. [DOI] [PubMed] [Google Scholar]
- 16.Hendrix, C. W., A. C. Collier, M. M. Lederman, D. Schols, R. B. Pollard, S. Brown, J. B. Jackson, R. W. Coombs, M. J. Glesby, C. W. Flexner, G. J. Bridger, K. Badel, R. T. MacFarland, G. W. Henson, and G. Calandra. 2004. Safety, pharmacokinetics, and antiviral activity of AMD3100, a selective CXCR4 receptor inhibitor, in HIV-1 infection. J. Acquir. Immune Defic. Syndr. 37:1253-1262. [DOI] [PubMed] [Google Scholar]
- 17.Ichiyama, K., S. Yokoyama-Kumakura, Y. Tanaka, R. Tanaka, K. Hirose, K. Bannai, T. Edamatsu, M. Yanaka, Y. Niitani, N. Miyano-Kurosaki, H. Takaku, Y. Koyanagi, and N. Yamamoto. 2003. A duodenally absorbable CXC chemokine receptor 4 antagonist, KRH-1636, exhibits a potent and selective anti-HIV-1 activity. Proc. Natl. Acad. Sci. U. S. A. 100:4185-4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaiser, D. W., J. T. Thurston, J. R. Dudley, F. C. Schaefer, I. Hechenbleikner, and D. Holmhansen. 1951. Cyanuric chloride derivatives. 2. Substituted melamines. J. Am. Chem. Soc. 73:2984-2986. [Google Scholar]
- 19.Ketas, T. J., S. M. Schader, J. Zurita, E. Teo, V. Polonis, M. Lu, P. J. Klasse, and J. P. Moore. 2007. Entry inhibitor-based microbicides are active in vitro against HIV-1 isolates from multiple genetic subtypes. Virology 364:431-440. [DOI] [PubMed] [Google Scholar]
- 20.Krebs, F. C., S. R. Miller, M. L. Ferguson, M. Labib, R. F. Rando, and B. Wigdahl. 2005. Polybiguanides, particularly polyethylene hexamethylene biguanide, have activity against human immunodeficiency virus type 1. Biomed. Pharmacother. 59:438-445. [DOI] [PubMed] [Google Scholar]
- 21.Krowicka, H., J. Robinson, R. Clark, S. Hager, S. Broyles, and S. Pincus. 2008. Use of tissue culture cell lines to evaluate HIV antiviral resistance. AIDS Res. Hum. Retrovir. 24:957-967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moyle, G., E. DeJesus, M. Boffito, R. Wong, C. Gibney, K. Badel, R. MacFarland, G. Calandra, G. Bridger, S. Becker, et al. 2009. Proof of activity with AMD11070, an orally bioavailable inhibitor of CXCR4-tropic HIV type 1. Clin. Infect. Dis. 48:798-805. [DOI] [PubMed] [Google Scholar]
- 23.Muller, D., I. Zeltser, G. Bitan, and C. Gilon. 1997. Building units for N-backbone cyclic peptides. 3. Synthesis of protected N-α-(omega-aminoalkyl)amino acids and N-α-(omega-carboxyalkyl) amino acids. J. Organic Chem. 62:411-416. [DOI] [PubMed] [Google Scholar]
- 24.Murakami, T., A. Yoshida, R. Tanaka, S. Mitsuhashi, K. Hirose, M. Yanaka, N. Yamamoto, and Y. Tanaka. 2004. KRH-2731: an orally bioavailable CXCR4 antagonist is a potent inhibitor of HIV-1 infection. Top. HIV Med. 12:541. [Google Scholar]
- 25.Narumi, T., R. Hayashi, K. Tomita, K. Kobayashi, N. Tanahara, H. Ohno, T. Naito, E. Kodama, M. Matsuoka, S. Oishi, and N. Fujii. 2010. Synthesis and biological evaluation of selective CXCR4 antagonists containing alkene dipeptide isosteres. Org. Biomol. Chem. 8:616-621. [DOI] [PubMed] [Google Scholar]
- 26.Rosenkilde, M. M., L. O. Gerlach, S. Hatse, R. T. Skerlj, D. Schols, G. J. Bridger, and T. W. Schwartz. 2007. Molecular mechanism of action of monocyclam versus bicyclam non-peptide antagonists in the CXCR4 chemokine receptor. J. Biol. Chem. 282:27354-27365. [DOI] [PubMed] [Google Scholar]
- 27.Seibert, C., and T. P. Sakmar. 2004. Small-molecule antagonists of CCR5 and CXCR4: a promising new class of anti-HIV-1 drugs. Curr. Pharm. Des. 10:2041-2062. [DOI] [PubMed] [Google Scholar]
- 28.Seibert, C., W. Ying, S. Gavrilov, F. Tsamis, S. E. Kuhmann, A. Palani, J. R. Tagat, J. W. Clader, S. W. McCombie, B. M. Baroudy, S. O. Smith, T. Dragic, J. P. Moore, and T. P. Sakmar. 2006. Interaction of small molecule inhibitors of HIV-1 entry with CCR5. Virology 349:41-54. [DOI] [PubMed] [Google Scholar]
- 29.Skerlj, R., G. Bridger, A. Kaller, E. McEachern, J. Crawford, Y. Zhou, B. Atsma, J. Langille, S. Nan, D. Veale, T. Wilson, C. Harwig, S. Hatse, K. Princen, E. De Clercq, and D. Schols. 2010. Discovery of novel small molecule orally bioavailable C-X-C chemokine receptor 4 antagonists that are potent inhibitors of T-tropic (X4) HIV-1 replication. J. Med. Chem. 53:3376-3388. [DOI] [PubMed] [Google Scholar]
- 30.Tamamura, H., K. Hiramatsu, S. Kusano, S. Terakubo, N. Yamamoto, J. O. Trent, Z. Wang, S. C. Peiper, H. Nakashima, A. Otaka, and N. Fujii. 2003. Synthesis of potent CXCR4 inhibitors possessing low cytotoxicity and improved biostability based on T140 derivatives. Org. Biomol. Chem. 1:3656-3662. [DOI] [PubMed] [Google Scholar]
- 31.Tamamura, H., M. Sugioka, Y. Odagaki, A. Omagari, Y. Kan, S. Oishi, H. Nakashima, N. Yamamoto, S. C. Peiper, N. Hamanaka, A. Otaka, and N. Fujii. 2001. Conformational study of a highly specific CXCR4 inhibitor, T140, disclosing the close proximity of its intrinsic pharmacophores associated with strong anti-HIV activity. Bioorg. Med. Chem. Lett. 11:359-362. [DOI] [PubMed] [Google Scholar]
- 32.Tamamura, H., Y. Xu, T. Hattori, X. Zhang, R. Arakaki, K. Kanbara, A. Omagari, A. Otaka, T. Ibuka, N. Yamamoto, H. Nakashima, and N. Fujii. 1998. A low-molecular-weight inhibitor against the chemokine receptor CXCR4: a strong anti-HIV peptide T140. Biochem. Biophys. Res. Commun. 253:877-882. [DOI] [PubMed] [Google Scholar]
- 33.Thakkar, N., V. Pirrone, S. Passic, S. Keogan, W. Zhu, V. Kholodovych, W. Welsh, R. Rando, M. Labib, B. Wigdahl, and F. Krebs. 2010. Persistent interactions between biguanide-based compound NB325 and CXCR4 result in prolonged inhibition of human immunodeficiency virus type 1 infection. Antimicrob. Agents Chemother. 54:1965-1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thakkar, N., V. Pirrone, S. Passic, W. Zhu, V. Kholodovych, W. Welsh, R. Rando, M. Labib, B. Wigdahl, and F. Krebs. 2009. Specific interactions between the viral coreceptor CXCR4 and the biguanide-based compound NB325 mediate inhibition of human immunodeficiency virus type 1 infection. Antimicrob. Agents Chemother. 53:631-638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsamis, F., S. Gavrilov, F. Kajumo, C. Seibert, S. Kuhmann, T. Ketas, A. Trkola, A. Palani, J. W. Clader, J. R. Tagat, S. McCombie, B. Baroudy, J. P. Moore, T. P. Sakmar, and T. Dragic. 2003. Analysis of the mechanism by which the small-molecule CCR5 antagonists SCH-351125 and SCH-350581 inhibit human immunodeficiency virus type 1 entry. J. Virol. 77:5201-5208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ueda, S., M. Kato, S. Inuki, H. Ohno, B. Evans, Z. X. Wang, S. C. Peiper, K. Izumi, E. Kodama, M. Matsuoka, H. Nagasawa, S. Oishi, and N. Fujii. 2008. Identification of novel non-peptide CXCR4 antagonists by ligand-based design approach. Bioorg. Med. Chem. Lett. 18:4124-4129. [DOI] [PubMed] [Google Scholar]
- 37.Ueda, S., S. Oishi, Z. X. Wang, T. Araki, H. Tamamura, J. Cluzeau, H. Ohno, S. Kusano, H. Nakashima, J. O. Trent, S. C. Peiper, and N. Fujii. 2007. Structure-activity relationships of cyclic peptide-based chemokine receptor CXCR4 antagonists: disclosing the importance of side chain and backbone functionalities. J. Med. Chem. 50:192-198. [DOI] [PubMed] [Google Scholar]
- 38.Vaillancourt, V. A., S. D. Larsen, S. P. Tanis, J. E. Burr, M. A. Connell, M. M. Cudahy, B. R. Evans, P. V. Fisher, P. D. May, M. D. Meglasson, D. D. Robinson, F. C. Stevens, J. A. Tucker, T. J. Vidmar, and J. H. Yu. 2001. Synthesis and biological activity of aminoguanidine and diaminoguanidine analogues of the antidiabetic/antiobesity agent 3-guanidinopropionic acid. J. Med. Chem. 44:1231-1248. [DOI] [PubMed] [Google Scholar]
- 39.Wagner, T. A., and L. M. Frenkel. 2008. Potential limitation of CCR5 antagonists: drug resistance more often linked to CXCR4-utilizing than to CCR5-utilizing HIV-1. AIDS 22:2393-2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Watson, C., S. Jenkinson, W. Kazmierski, and T. Kenakin. 2005. The CCR5 receptor-based mechanism of action of 873140, a potent allosteric noncompetitive HIV entry inhibitor. Mol. Pharmacol. 67:1268-1282. [DOI] [PubMed] [Google Scholar]
- 41.Wei, X., J. Decker, H. Liu, Z. Zhang, R. Arani, J. Kilby, M. Saag, X. Wu, G. Shaw, and J. Kappes. 2002. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob. Agents Chemother. 46:1896-1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.