Abstract
Cytomegalovirus (CMV) infection is the leading cause of congenital infection, producing both sensorineural hearing loss and mental retardation. We evaluated the in vivo efficacy of an orally bioavailable analog of cidofovir, hexadecyloxypropyl-cidofovir (HDP-CDV), against guinea pig CMV (GPCMV) in a guinea pig model of congenital CMV infection. HDP-CDV exhibited antiviral activity against GPCMV with a 50% effective concentration (EC50) of 0.004 μM ± 0.001 μM. To evaluate in vivo efficacy, pregnant Hartley guinea pigs were inoculated with GPCMV during the late second/early third trimester of gestation. Animals were administered 20 mg HDP-CDV/kg body weight orally at 24 h postinfection (hpi) and again at 7 days postinfection (dpi) or administered 4 mg/kg HDP-CDV orally each day for 5 days or 9 days. Virus levels in dam and pup tissues were evaluated following delivery, or levels from dam, placenta, and fetal tissues were evaluated following sacrifice of dams at 10 dpi. All HDP-CDV regimens significantly improved pup survival, from 50 to 60% in control animals to 93 to 100% in treated animals (P ≤ 0.019). Treatment with 20 mg/kg HDP-CDV significantly reduced the viral load in pup spleen (P = 0.017) and liver (P = 0.029). Virus levels in the placenta were significantly reduced at 10 dpi following daily treatment with 4 mg/kg HDP-CDV for 5 or 9 days. The 9-day treatment also significantly reduced the viral levels in the dam spleen and liver. Although the 4-mg/kg treatment improved pup survival, virus levels in the fetal tissues were similar to those in control tissues. Taken together, HDP-CDV shows potential as a well-tolerated antiviral candidate for treatment of congenital human CMV (HCMV) infection.
Cytomegalovirus (CMV) is a betaherpesvirus that is the leading cause of congenital infection worldwide, occurring in 1 to 2.5% of all newborns in the developed world (37, 41). Approximately 40,000 cases are seen each year in the United States (17, 18). Although the majority of congenitally infected newborns are asymptomatic, approximately 10% of the newborns develop mild to severe primary disease (42). Neurologic defects and deafness are the most important sequelae in survivors. Importantly, 7 to 25% of babies asymptomatic at birth will exhibit central nervous system (CNS) deficits and progressive deafness later in life (16, 25), making CMV the most common cause of infectious deafness.
Although progress in understanding the consequences of congenital CMV infection has been made, prevention of congenital infection has not been achieved and management of newborns with congenital CMV infection is limited. Most recently, treatment of symptomatic CMV-infected infants with intravenous (i.v.) ganciclovir for 6 weeks was found effective in reducing the progression of deafness for at least 6 to 12 months (29, 30) and resulted in fewer developmental delays at 6 and 12 months than in untreated infants (39). Oral valganciclovir treatment given alone or following intravenous ganciclovir was also recently found to reduce urine and plasma levels in newborns with symptomatic congenital CMV infection (1, 20, 29, 32-35).
Other approaches to preventing or improving the outcome of congenital CMV are aimed at the mother. These include vaccines (43) and treatments of CMV-infected pregnant women with either CMV hyperimmune globulin (38) or antivirals. Several recent reports that demonstrate treatment of pregnant women with CMV-infected fetuses with oral ganciclovir, intravenous ganciclovir (7, 44), or valacyclovir (26) have been published. However, the toxicity and teratogenicity of ganciclovir or valganciclovir as well as other currently available anti-CMV drugs limit the use of antiviral therapy for either pregnant women or congenitally infected infants. Thus, further evaluations of drugs that are safer and more effective against CMV when given orally are warranted.
Guinea pig cytomegalovirus (GPCMV) is the only small-animal cytomegalovirus that crosses the placenta, similarly to human CMV (HCMV) infections. Therefore, this model has been used by us and others to study the pathogenesis and treatment of congenital CMV infection (4, 8, 9, 22, 31). In the studies reported here, we evaluated the in vivo antiviral activity of hexadecyloxypropyl-cidofovir (HDP-CDV) in the guinea pig model of congenital CMV infection. HDP-CDV is an alkoxyalkyl ester analog of cidofovir (CDV) that exhibits enhanced in vitro activity against HCMV and other herpesviruses and has been shown to be active in vivo against HCMV and murine CMV (MCMV) in animal models (3, 5, 24, 27, 45, 48). HDP-CDV is also active against a range of orthopoxviruses, adenoviruses, and other double-stranded DNA viruses (24, 40, 46, 47). HDP-CDV differs from CDV in that the drug is orally bioavailable, with a relatively long half-life both in the plasma and within cells (5, 10, 24). Pharmacokinetic evaluation of HDP-CDV in mice indicates 93% oral bioavailability, compared to 5% for CDV (10). Importantly, because of the alkoxyalkyl esterification, HDP-CDV does not accumulate in renal tubular cells and thus lacks nephrotoxicity, as opposed to CDV (10, 24). Although the pharmacokinetics of HDP-CDV in guinea pigs is not known, a number of species, including mice, rats, rabbits, and humans, have been evaluated for bioavailability and antiviral activity of cyclic 1-[((S)-2-hydroxy-2-oxo-1,4,2-dioxaphosphorinan-5-yl)methyl]cytosine (cHPMPC) and its parent compound CDV against herpesviruses (12-14, 49).
In this paper, we present the first evaluation of HDP-CDV against congenital CMV infection. We evaluated the impact of HDP-CDV on pup mortality and viral transmission to the developing fetus and the activity of HDP-CDV against viral levels in dam tissues, fetal tissues, and tissues of newborn pups shortly after birth. HDP-CDV, also known as CMX001 (24), is currently in phase 1/phase 2 clinical development and shows potential as an oral drug for the treatment and prevention of congenital CMV infections and associated complications.
(The data provided in the study were presented at the following meetings: the 21st International Conference on Antiviral Research, 13 to 17 April 2008, Montreal, Quebec, Canada; the 33rd International Herpesvirus Workshop, 27 July to 1 August 2008, Estoril, Portugal; and the 2nd Congenital CMV Conference, 2 to 5 November 2008, CDC, Atlanta, GA.)
MATERIALS AND METHODS
Virus.
Guinea pig CMV (GPCMV, strain 22122; American Type Culture Collection, Manassas, VA) stock was prepared by sequential in vivo passage in male strain 2 guinea pigs as described previously (9). A salivary gland virus stock (passage 11) was used for all experiments. Guinea pig lung fibroblast monolayers (GPL cell line CCL-158; American Type Culture Collection, Manassas, VA) were grown and maintained with F-12 medium (Invitrogen Corporation, Carlsbad, CA) supplemented with 10% fetal bovine serum (HyClone, Logan, UT) and penicillin-streptomycin, 10,000 U/ml (Invitrogen Corporation, Carlsbad, CA).
Guinea pigs.
Pregnant Hartley strain guinea pigs at ∼35 to 45 days of gestation (of a 65- to 70-day gestation) were obtained initially from GPCMV-free colonies at Harlan Laboratories (Indianapolis, IN) and later from the GPCMV-free colony originally established from these animals and maintained at Cincinnati Children's Hospital Research Foundation, Cincinnati, OH, when these guinea pigs were no longer available. Animals were housed in facilities approved by the Association for Assessment and Accreditation of Laboratory Animal Care International, and all procedures were approved by the Institutional Animal Care and Use Committee.
Antiviral compound.
Hexadecyloxypropyl-cidofovir (HDP-CDV) was synthesized and characterized as previously described (28), with a purity of >98%. The structures of CDV and HDP-CDV are shown in Fig. 1. HDP-CDV was prepared in saline solution and administered orally to guinea pigs as previously described (24, 27). For in vitro antiviral assays, HDP-CDV was dissolved in saline solution.
FIG. 1.
Structures of CDV and HDP-CDV.
In vitro antiviral assay.
For the plaque reduction assay, 100 PFU of GPCMV was mixed with HDP-CDV ranging from 0.0001 μM to 10 μM and adsorbed onto GPL cells for 1 h. The same concentration of compound was also included in the methylcellulose overlay, followed by a 10-day incubation. The assays were performed in duplicate wells in three separate experiments. The 50% effective concentration (EC50) for HDP-CDV was calculated as the concentration of compound which reduced the number of plaques by 50% compared to that for the untreated control. Cytotoxicity of compounds was assessed following crystal violet staining of uninfected guinea pig fibroblasts that were incubated for 10 days with increasing concentrations of HDP-CDV ranging from 1.0 μM to 100 μM and compared microscopically to control monolayers that were not exposed to compound.
Experimental infection and tissue collection.
Pregnant guinea pigs were inoculated subcutaneously (s.c.) with 1 × 105 PFU of GPCMV (0.5-ml volume) between the second and third trimesters of pregnancy (∼day 45 to 50 of pregnancy) (9). Pregnant guinea pigs were then treated by oral gavage (1.0-ml volume) with HDP-CDV or saline solution as a control. In the first experiment, dams were treated orally with 20 mg HDP-CDV/kg body weight at 24 h postinfection (hpi) with HDP-CDV or saline and again at 7 days postinfection (dpi). In the second experiment, dams were treated orally with 4 mg/kg HDP-CDV or saline at 24 hpi and then once daily for 5 days. In both experiments, the dams were observed daily and were sacrificed within 1 week following delivery of pups. Tissues from stillborn pups were collected immediately after delivery, and specimens of liver and spleen were stored for PCR analysis of GPCMV DNA. Live-born pups were sacrificed within 3 to 7 days of delivery, and the liver and spleen were obtained for PCR analysis of viral loads. Viral DNA levels in the dam spleen and liver were also determined by PCR analysis. In the next set of experiments, animals were infected as described above and treated with 4 mg/kg HDP-CDV once daily for 5 days or 9 days. In these studies, the dams were sacrificed at 10 dpi for evaluation of GPCMV in the placenta, liver, and spleen of the dam and liver and spleen of the fetus. This time was selected because it is a time before delivery is expected in the control dams and at which ∼100% of control placentas are infected (9). All samples were frozen at −80°C for later evaluation by PCR. To evaluate viremia, blood samples were obtained by toenail clipping from dams on 5, 7, and 10 dpi and analyzed by PCR.
To further assess safety, uninfected dams (n = 3) were treated with 4 mg/kg HDP-CDV or saline daily for 9 days. Prior to treatment, blood was collected to assess baseline values. Blood was next collected from dams at 10 days and from newborn pups (n = 9) born to treated and untreated dams within 3 days after birth for evaluation of bone marrow (complete blood count), liver (bilirubin, alanine aminotransferase [ALT], and aspartate transaminase [AST] concentrations), and kidney (blood urea nitrogen and creatinine concentrations and serum chemistries) toxicity. In addition, pup weights at birth were obtained to assess whether treatment affected fetal growth or development.
DNA extraction and real-time PCR.
DNA extraction and quantitative PCR analysis were performed as previously described (9). Briefly, DNA was extracted from 200 μl of the 10% homogenate (representing 20 mg of tissue) by use of a Qiagen QIAamp minikit DNA extraction system, according to manufacturer's instructions (Qiagen, Inc., Valencia, CA). DNA was also isolated from 200 μl of blood collected by toenail bleeds. GPCMV DNA, extracted from a salivary gland-derived virus stock, was used as a positive control, and uninfected guinea pig spleen DNA was used as a negative control. To quantify viral loads, UL97F1/R1 primers were used to evaluate samples with LightCycler real-time PCR SYBR green I fluorescent dye (Roche, Indianapolis, IN). The primers UL97F1 (5′ GATCGCTTCTGTCAACACG 3′) and UL97R1 (5′ CGCAACTGATCGAATATCCTG 3′) amplify a 100-bp region of the GPCMV UL97 gene. The plasmid pFB97, which encodes a 306-bp region of the UL97 DNA gene, was used to generate a standard curve by serial 10-fold dilution of pFB97 DNA (1 × 100 to 1 × 105 plasmid copies) in 50 ng of uninfected guinea pig spleen DNA. Controls included uninfected guinea pig DNA and a no-DNA template. FastStart DNA MasterPlus SYBR green I reaction mix (Roche, Indianapolis, IN) containing primers (1 μmol/liter) and nuclease-free water was employed for all reactions. Samples (5 μl) containing either 50 or 100 ng of eluted DNA were added for a total reaction volume of 20 μl. The following cycling program was used: 95°C for 10 min, followed by 50 cycles of 10 s at 95°C, 5 s at 59°C, and 5 s at 72°C. For negative reactions, a 1:10 dilution of the samples was retested to eliminate the possibility of PCR inhibition. The limit of detection of the assay was between 1 and 10 copies, with a faint band detectable at 1 copy in some experiments. For statistical comparisons, negative samples were assigned a copy number of 1 (corresponding to 1.3 log10 per μg of DNA). Amplification products were examined by 2% agarose gel electrophoresis (Invitrogen Corporation).
Statistics.
The means ± standard deviations of viral DNA levels were compared by Student's t test. Mortality rates were compared by Fisher's exact test. All comparisons were two-tailed. Tests were performed using GraphPad InStat version 3.05 for Windows (GraphPad Software, San Diego, CA).
RESULTS
HDP-CDV exhibits potent in vitro antiviral activity against guinea pig cytomegalovirus.
It was previously reported that HDP-CDV exhibits approximately 30- to 300-fold-greater activity than CDV against HCMV, MCMV, and GPCMV (3, 27). We first evaluated the in vitro activity of HDP-CDV against GPCMV during infection of guinea pig lung fibroblasts. HDP-CDV demonstrated approximately 78-fold-greater activity than CDV against GPCMV, with an EC50 of 0.004 ± 0.001 μM, similar to the previously established EC50s of HDP-CDV against HCMV and MCMV (3, 27). Similarly, HDP-CDV did not exhibit toxicity on guinea pig fibroblasts, with a 50% cytotoxic concentration (CC50) of >100 μM and a selective index (CC50/EC50) of >25,000.
Effect of HDP-CDV treatment on pregnancy outcome and GPCMV infection.
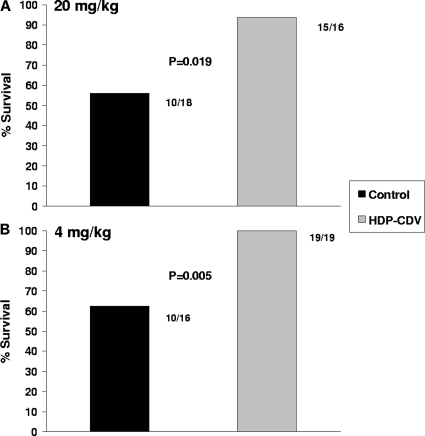
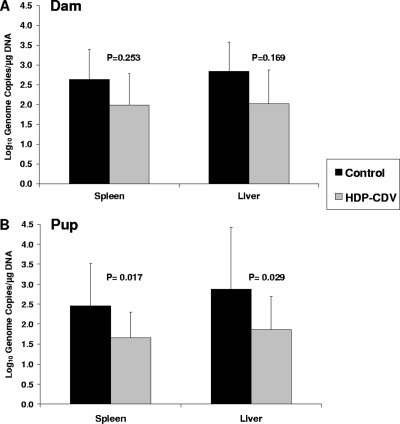
The aim of the first set of experiments was to evaluate the effect of maternal HDP-CDV treatment on pregnancy outcome following GPCMV infection. As seen in Fig. 2A, oral treatment of pregnant guinea pigs with 20 mg/kg HDP-CDV at 24 hpi and at 7 dpi significantly increased the percentage of live-born pups, from 55.6% (10/18) in the control group to 93.8% (15/16) in the HDP-CDV-treated group (P = 0.019). Further, virus levels in the blood were significantly reduced at 7 dpi in the treated dams (2.88 ± 0.10 log10 genome copies/μg DNA) compared to those in the control dams (3.22 ± 0.2 log10 genome copies/μg DNA) (P = 0.006). However, although viral DNA levels in the treated dams were 5- and 6.5-fold lower in the spleen and liver, respectively, than those in the control dams, this did not achieve statistical significance (Fig. 3A). In contrast, as shown in Fig. 3B, treatment of the dams significantly reduced viral DNA levels in the pup spleens (∼6-fold, P = 0.017) and pup livers (∼10.5-fold, P = 0.029) compared to those in pup tissues from untreated dams. The percentage of pup tissues with detectable viral DNA was also lower in the pups born to treated dams than in the pups born to untreated dams (25% versus 44%, respectively, in the spleen and 57% versus 73%, respectively, in the liver, although not significantly different). These data indicate that although most pups were infected, oral treatment of the dams with 20 mg/kg HDP-CDV at 24 hpi and at 7 dpi improved pup survival, reduced the numbers of pups with detectable viral DNA, and significantly reduced the viral DNA levels in the pup tissues.
FIG. 2.
Survival of guinea pig pups following HDP-CDV treatment of pregnant guinea pigs. Dams were challenged with GPCMV during middle to late gestation. (A) Dams were treated with 20 mg/kg HDP-CDV (n = 4) or saline (n = 5) at 24 hpi and 7 dpi. (B) Dams were treated with 4 mg/kg HDP-CDV (n = 4) or saline (n = 5) for 5 days. The percent survival and the numbers of pups which survived are shown. P values are depicted, and data represent results from one study each.
FIG. 3.
Real-time PCR analysis of GPCMV DNA loads in tissues from dams and pups sacrificed within 7 days of birth, following HDP-CDV treatment. Dams were treated with 20 mg/kg HDP-CDV (n = 4) or saline (n = 5) at 24 hpi and 7 dpi. Tissues from dams and control pup tissue samples (n = 15) and treated pup tissue samples (n = 16) were evaluated by PCR. Data represent the mean viral genome copies ± standard deviations from one study. P values are depicted.
To further examine the effects of therapy on the outcome of pup survival, continuous daily dosing was evaluated in a second study in which pregnant guinea pigs were treated with a lower dose of HDP-CDV, 4 mg/kg, for 5 consecutive days after infection, again beginning at 24 hpi. As seen in Fig. 2B, this regimen significantly increased the percentage of live-born pups, from 62.5% (10/16) in the control group to 100% (19/19) in the HDP-CDV-treated group (P = 0.005).
Lastly, both treatment regimens, two doses of 20 mg/kg HDP-CDV and 5 days of consecutive treatment with 4 mg/kg HDP-CDV, appeared to be well tolerated in the infected pregnant guinea pigs, since none of the pregnant animals exhibited signs of toxicity, such as decreased activity or obvious weight loss. Similarly, newborn pups from treated dams did not exhibit low birth weights or other obvious signs of toxicity within the first week after birth. The mean birth weights for pups born to treated dams (n = 12 pups) and untreated dams (n = 9 pups) were 103 ± 19 g and 107 ± 10 g, respectively (P = 0.567). One treated dam delivered three stillborn pups and one live pup, with two of the stillborn pups abnormally large (130 and 138 g). Overall, ∼91% of pups (43/47) were delivered live to dams which were treated with HDP-CDV in these studies. To further evaluate the safety of HDV-CDV in uninfected dams and unborn pups, samples were collected before and after dams were treated with 4 mg/kg HDP-CDV daily for 9 days. HDP-CDV treatment had no adverse effects on complete blood counts in either the treated dams (n = 3) or the newborn pups (n = 9) (data not shown), and blood counts were similar to those obtained from untreated dams and newborn pups. In addition, complete liver and kidney profiles indicated no detectable liver or kidney toxicity in either treated dams or their newborn pups and were similar to those from untreated dams and their newborn pups (data not shown).
Effect of HDP-CDV treatment on viral load in dams and fetuses during gestation.
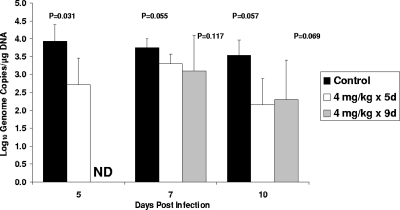
We next examined the effects of HDP-CDV therapy for 5 days or 9 days on virus replication in the dam, placenta, and fetuses prior to delivery (9). In this study, animals were sacrificed at 10 days after virus infection, a time before delivery is expected in the control dams and at which ∼100% of control placentas are infected (9). As a measure of the efficacy of daily oral treatment with 4 mg/kg HDP-CDV, viremia was analyzed. A significant reduction of viremia in the treated dams was detected only at 5 dpi (Fig. 4). Furthermore, as shown in Table 1, 5 days of therapy did not reduce the number of dams with detectable viral DNA (100% for both the treated and untreated dams) but did reduce viral DNA levels in the dam tissues. In dams treated with HDP-CDV for 5 days, the viral DNA load in the livers was reduced ∼69-fold compared to the viral DNA load detected in the control livers (P = 0.05). Although not significant, the viral DNA detected in spleens from dams treated for 5 days was also reduced, ∼22-fold compared to that in spleens from the control dams (P = 0.067). When virus replication in the placentas was evaluated, there was also no effect on the percentage of infected placentas (100% for both groups), but the viral load in the drug-treated placentas was significantly reduced, ∼5-fold compared to that in the control placentas (P = 0.041).
FIG. 4.
Real-time PCR analysis of GPCMV viremia in dams treated with HDP-CDV. Viremia in animals treated with 4 mg/kg HDP-CDV for 5 days (4 mg/kg × 5d; n = 4) or 9 days (4 mg/kg × 9d; n = 5) or with saline (n = 4) is shown. Data represent the mean viral genome copies ± standard deviations from one study. P values are depicted. ND, samples were not collected.
TABLE 1.
Detection of GPCMV in dam and fetal organs by real-time PCR at 10 days postinoculationa
| Group and treatment regimenb | Liver |
Spleen |
Placenta |
|||
|---|---|---|---|---|---|---|
| No. positive/total no. (%) | Viral load (P value) | No. positive/total no. (%) | Viral load (P value) | No. positive/total no. (%) | Viral load (P value) | |
| Dam | ||||||
| Control | 4/4 (100) | 4.82 ± 0.8 | 4/4 (100) | 4.12 ± 0.3 | 15/15 (100) | 3.40 ± 0.7 |
| HDP-CDV 4 mg/kg × 5d | 4/4 (100) | 2.98 ± 1.3 (0.050) | 4/4 (100) | 2.77 ± 1.2 (0.067) | 13/13 (100) | 2.70 ± 0.9 (0.041) |
| HDP-CDV 4 mg/kg × 9d | 5/5 (100) | 3.01 ± 1.0 (0.024) | 5/5 (100) | 2.09 ± 0.5 (<0.001) | 13/13 (100) | 2.75 ± 0.5 (0.009) |
| Fetus | ||||||
| Control | 13/15 (87) | 2.20 ± 0.6 | 7/15 (47) | 1.63 ± 0.6 | ||
| HDP-CDV 4 mg/kg × 5d | 12/13 (92) | 2.12 ± 0.7 (0.265) | 10/13 (77) | 1.91 ± 0.6 (0.218) | ||
| HDP-CDV 4 mg/kg × 9d | 11/13 (85) | 1.87 ± 0.6 (0.752) | 8/13 (62) | 1.65 ± 0.5 (0.563) | ||
Viral load values represent the mean log10 genome copies/μg DNA ± standard deviations. All P values compare treated and control groups. Significant P values are shown in bold. Data shown are from one study each.
HDP-CDV 4 mg/kg × 5d, treatment with 4 mg/kg HDP-CDV for 5 days; HDP-CDV 4 mg/kg × 9d, treatment with 4 mg/kg HDP-CDV for 9 days.
In the last evaluation, treatment with 4 mg/kg of HDP-CDV was extended to 9 days. This regimen also did not reduce the number of infected animals (100% of dams infected) but did reduce the viral load in the tissues. In the spleen, the viral load was reduced ∼100-fold in the treated dams (P < 0.001). In the liver, the viral load was also significantly reduced, ∼65-fold, in the treated dams (P = 0.024) (Table 1). These results indicate that extending the treatment time to 9 days had a greater impact on viral DNA levels in the dam tissues, especially in the spleen. Lastly, viral DNA loads in the placentas were evaluated following the 9-day treatment period. As shown in Table 1, 100% of the placentas were infected in both the control dams (15/15) and the dams treated for 9 days (13/13). However, viral DNA levels in the placentas were significantly reduced ∼4.5-fold in the treated dams compared to viral DNA levels in control placentas (P = 0.009).
To determine the effects of treatment on viral replication in fetal tissues at 10 dpi, we quantified viral DNA levels in the fetal livers and spleens from HDP-CDV-treated and control groups (Table 1). Unexpectedly, there was no significant difference in number of infected fetal tissues or viral load in the livers or spleens from fetuses of the treated dams compared to those of the control dams. Thus, the treatment of pregnant guinea pigs with a lower dose of HDP-CDV for 5 or 9 consecutive days did not prevent or limit infection of the fetuses, even though viral load in the placentas was significantly reduced and pup survival was greatly improved.
In summary, these experiments demonstrate that oral HDP-CDV treatment of GPCMV-infected pregnant guinea pigs improves the outcome of congenital infection, although at the dose regimens examined here, it did not prevent infection of the placenta or infection of the fetuses. The impact of therapy on viral replication in the dam, newborn pup, and fetal tissues appeared to depend on the dosing regimen of HDP-CDV. Overall, treatment with HDP-CDV appears to be well tolerated in the pregnant guinea pig and thus is expected to be a potential new antiviral candidate for the treatment of congenital CMV infection and associated complications.
DISCUSSION
Congenital CMV infections in newborns are recognized as a significant public health issue, and the development of a successful vaccine and/or the development of safe and well-tolerated antiviral agents remains a priority. Although no vaccine is currently available, there are four licensed antivirals for the systemic treatment of CMV: ganciclovir, valganciclovir (oral prodrug of ganciclovir), foscarnet, and cidofovir (36). However, these antivirals exhibit numerous drug toxicities, thus limiting their use for treatment of congenital CMV infection in pregnant women or newborns (reviewed in reference 6).
In the studies reported here, we evaluated the antiviral efficacy of HDP-CDV on congenital CMV infection by using the guinea pig model of congenital CMV. HDP-CDV is a lipid ester analog of CDV that exhibits enhanced potency against a number of herpesviruses and, most significantly, lacks the renal toxicity of CDV (24). Similarly to the increased antiviral activity of HDP-CDV against HCMV (3, 48), our studies show that HDP-CDV also exhibited increased antiviral activity against GPCMV compared to that of CDV.
Previously, we demonstrated that antiviral treatment of pregnant GPCMV-infected guinea pigs with one dose of 35 mg/kg cHPMPC, a cyclic analog of CDV, resulted in significantly improved pup survival and decreased placental and fetal tissue infection (9). Similarly, as shown in our studies presented here, HDP-CDV is active against GPCMV infection in the pregnant guinea pig following oral administration. In our first study, two doses of 20 mg/kg HDP-CDV administered 24 h and 7 days after GPCMV infection significantly decreased maternal viremia by 7 dpi but did not prevent viremia. Most importantly, pup survival was significantly increased. Evaluation of the viral load in the treated dam following pup delivery showed that the 20 mg/kg HDP-CDV treatment did not significantly decrease the viral titers detected in the dam liver and spleen. This is most likely due to “rebound virus replication,” since samples were obtained ∼20 days after the second drug treatment. This suggests that sustained HDP-CDV levels in the dam following the last treatment at 7 dpi were inadequate to significantly maintain decreased GPCMV replication in the infected dam tissues. However, in the newborn pups, the viral load was significantly reduced in the spleen and liver, indicating that two doses of 20 mg/kg HDP-CDV had either limited the infection of the placenta and thus transmission to the fetus or limited the virus replication in fetal tissues directly. Indeed, in subsequent experiments with lower doses, we found that HDP-CDV decreased virus replication in the placenta but not the fetal tissue, as discussed below.
To further evaluate HDP-CDV antiviral activity following oral delivery, we treated the dams for 5 or 9 consecutive days beginning 24 h after infection with a lower dose of HDP-CDV, 4 mg/kg. Similar to the first study, dam viremia was decreased while pup survival increased to 100%. However, the 4 mg/kg HDP-CDV treatment did not result in reduced viremia after 5 dpi. When animals were sacrificed at 10 dpi, we found that treatment did not prevent infection of the placenta or fetus but that viral replication in the placenta was significantly decreased following 5 or 9 days of therapy. In contrast to treatment with 20 mg/kg HDP-CDV, which reduced viral replication in newborn tissues, treatment with 4 mg/kg for either 5 or 9 consecutive days did not reduce viral levels in fetal tissues. It is possible that doses higher than 4 mg/kg of HDP-CDV or longer treatment durations are needed to limit or prevent viral infection of the fetal tissues.
Our safety analysis included laboratory evaluation of a limited number of dams and their pups, including analysis of pup size. These evaluations indicated that the HDP-CDV treatment regimen utilized in our studies was not toxic to the pregnant animal or the developing fetus. Only 4 of the 47 pups which were delivered to treated dams in our studies (3 pups in the safety study and 1 pup in the 20-mg/kg HDP-CDV study) did not survive, and none of the remaining 43 pups exhibited adverse symptoms of toxicity. It is unclear why one treated dam delivered two abnormally large pups and one smaller pup that did not survive to full term.
In humans, CMV infection of the placenta without infection of the fetus is thought to be common and may result in spontaneous abortion (11, 15, 19, 21, 23). In addition, the pathogenesis of congenital infection is not completely understood. A growing body of evidence suggests that some symptoms of congenital CMV infection could be due to the indirect consequences of placental dysfunction caused by infection of the placenta rather than infection of the fetus (reviewed in reference 2). Intrauterine hypoxemia or placental insufficiency and/or fetal hypoxia would result in developmental impairment of the fetus. In our studies, treatment with 4 mg/kg HDP-CDV provided 100% protection from fetal death and significantly reduced the viral load in the placenta at 10 dpi but did not significantly decrease the viral levels in the fetal tissues. This low-dose treatment regimen thus could have provided sufficient protection to the fetus by limiting the viral load in the placenta and preventing placental impairment, leading to increased pup survival, while the 20 mg/kg dose achieved levels sufficient to also reduce virus replication in the fetus. Thus, lower HDP-CDV doses may not have crossed the placenta-fetus barrier at levels high enough to significantly reduce viral DNA levels in the fetal spleens and livers. It is predicted that a higher dose of HDP-CDV for 5 to 9 days would have decreased the viral load in the fetuses at 10 dpi. Further studies are needed to determine whether alternative dosing regimens of HDP-CDV will prevent or limit infection of the placenta and/or fetuses in this model.
In conclusion, we have demonstrated that oral administration of HDP-CDV to pregnant dams is highly efficacious, significantly increasing pup survival and decreasing viral replication in both dam and pup tissues, depending on the treatment regimen. HDP-CDV was well tolerated in pregnant guinea pigs and did not appear to adversely affect fetal development. These data strongly indicate that HDP-CDV, an orally bioavailable drug, is a potent and well-tolerated antiviral candidate for treatment of congenital CMV infection.
Acknowledgments
K. Y. Hostetler has an equity interest and serves as a consultant to Chimerix, Inc., the licensee of HDP-CDV (CMX001). The terms of this arrangement have been reviewed and approved by the University of California San Diego in accordance with its conflict-of-interest policies.
Financial support was provided by National Institutes of Health contract no. AI-15439 and by NIAID grant AI-074057 (the latter to K.Y.H.).
Footnotes
Published ahead of print on 15 November 2010.
REFERENCES
- 1.Acosta, E. P., R. C. Brundage, J. R. King, P. J. Sanchez, S. Sood, V. Agrawal, J. Homans, R. F. Jacobs, D. Lang, J. R. Romero, J. Griffin, G. Cloud, R. Whitley, and D. W. Kimberlin. 2007. Ganciclovir population pharmacokinetics in neonates following intravenous administration of ganciclovir and oral administration of a liquid valganciclovir formulation. Clin. Pharmacol. Ther. 81:867-872. [DOI] [PubMed] [Google Scholar]
- 2.Adler, S. P., G. Nigro, and L. Pereira. 2007. Recent advances in the prevention and treatment of congenital cytomegalovirus infections. Semin. Perinatol. 31:10-18. [DOI] [PubMed] [Google Scholar]
- 3.Beadle, J. R., C. Hartline, K. A. Aldern, N. Rodriguez, E. Harden, E. R. Kern, and K. Y. Hostetler. 2002. Alkoxyalkyl esters of cidofovir and cyclic cidofovir exhibit multiple-log enhancement of antiviral activity against cytomegalovirus and herpesvirus replication in vitro. Antimicrob. Agents Chemother. 46:2381-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bia, F. J., B. P. Griffith, C. K. Fong, and G. D. Hsiung. 1983. Cytomegaloviral infections in the guinea pig: experimental models for human disease. Rev. Infect. Dis. 5:177-195. [DOI] [PubMed] [Google Scholar]
- 5.Bidanset, D. J., J. R. Beadle, W. B. Wan, K. Y. Hostetler, and E. R. Kern. 2004. Oral activity of ether lipid ester prodrugs of cidofovir against experimental human cytomegalovirus infection. J. Infect. Dis. 190:499-503. [DOI] [PubMed] [Google Scholar]
- 6.Biron, K. K. 2006. Antiviral drugs for cytomegalovirus diseases. Antiviral Res. 71:154-163. [DOI] [PubMed] [Google Scholar]
- 7.Brady, R. C., M. R. Schleiss, D. P. Witte, et al. 2002. Placental transfer of ganciclovir in a woman with acquired immunodeficiency syndrome and cytomegalovirus disease. Pediatr. Infect. Dis. J. 21:796-797. [DOI] [PubMed] [Google Scholar]
- 8.Bratcher, D. F., N. Bourne, F. J. Bravo, M. R. Schleiss, M. Slaoui, M. G. Myers, and D. I. Bernstein. 1995. Effect of passive antibody on congenital cytomegalovirus infection in guinea pigs. J. Infect. Dis. 172:944-950. [DOI] [PubMed] [Google Scholar]
- 9.Bravo, F. J., R. D. Cardin, and D. I. Bernstein. 2006. Effect of maternal treatment with cyclic HPMPC in the guinea pig model of congenital cytomegalovirus infection. J. Infect. Dis. 193:591-597. [DOI] [PubMed] [Google Scholar]
- 10.Ciesla, S. L., J. Trahan, W. B. Wan, J. R. Beadle, K. A. Aldern, G. R. Painter, and K. Y. Hostetler. 2003. Esterification of cidofovir with alkoxyalkanols increases oral bioavailability and diminishes drug accumulation in kidney. Antiviral Res. 59:163-171. [DOI] [PubMed] [Google Scholar]
- 11.Cruz Spano, L., F. E. Lima Pereira, N. Gomes da Silva Basso, and P. R. Mercon-de-Varga. 2002. Human cytomegalovirus infection and abortion: an immunohistochemical study. Med. Sci. Monit. 8:BR230-BR235. [PubMed] [Google Scholar]
- 12.Cundy, K. C., P. Barditch-Crovo, B. G. Petty, A. Ruby, M. Redpath, H. S. Jaffe, and P. S. Lietman. 1999. Clinical pharmacokinetics of 1-[((S)-2-hydroxy-2-oxo-1,4,2-dioxaphosphorinan-5-yl)methyl]cytosine in human immunodeficiency virus-infected patients. Antimicrob. Agents Chemother. 43:271-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cundy, K. C., A. M. Bidgood, G. Lynch, J. P. Shaw, L. Griffin, and W. A. Lee. 1996. Pharmacokinetics, bioavailability, metabolism, and tissue distribution of cidofovir (HPMPC) and cyclic HPMPC in rats. Drug Metab. Dispos. 24:745-752. [PubMed] [Google Scholar]
- 14.Cundy, K. C., G. Lynch, J. P. Shaw, M. J. Hitchcock, and W. A. Lee. 1996. Distribution and metabolism of intravitreal cidofovir and cyclic HPMPC in rabbits. Curr. Eye Res. 15:569-576. [DOI] [PubMed] [Google Scholar]
- 15.Fisher, S., O. Genbacev, E. Maidji, and L. Pereira. 2000. Human cytomegalovirus infection of placental cytotrophoblasts in vitro and in utero: implications for transmission and pathogenesis. J. Virol. 74:6808-6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fowler, K. B., F. P. McCollister, A. J. Dahle, S. Boppana, W. J. Britt, and R. F. Pass. 1997. Progressive and fluctuating sensorineural hearing loss in children with asymptomatic congenital cytomegalovirus infection. J. Pediatr. 130:624-630. [DOI] [PubMed] [Google Scholar]
- 17.Freij, B. J., and J. L. Sever. 1988. Herpesvirus infections in pregnancy: risks to embryo, fetus, and neonate. Clin. Perinatol. 15:203-231. [PubMed] [Google Scholar]
- 18.Gaytant, M. A., E. A. Steegers, B. A. Semmekrot, H. M. Merkus, and J. M. Galama. 2002. Congenital cytomegalovirus infection: review of the epidemiology and outcome. Obstet. Gynecol. Surv. 57:245-256. [DOI] [PubMed] [Google Scholar]
- 19.Griffith, B. P., S. R. McCormick, C. K. Fong, J. T. Lavallee, H. L. Lucia, and E. Goff. 1985. The placenta as a site of cytomegalovirus infection in guinea pigs. J. Virol. 55:402-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Griffiths, P. D. 2002. Strategies to prevent CMV infection in the neonate. Semin. Neonatol. 7:293-299. [DOI] [PubMed] [Google Scholar]
- 21.Griffiths, P. D., and C. Baboonian. 1984. A prospective study of primary cytomegalovirus infection during pregnancy: final report. Br. J. Obstet. Gynaecol. 91:307-315. [DOI] [PubMed] [Google Scholar]
- 22.Harrison, C. J., W. J. Britt, N. M. Chapman, J. Mullican, and S. Tracy. 1995. Reduced congenital cytomegalovirus (CMV) infection after maternal immunization with a guinea pig CMV glycoprotein before gestational primary CMV infection in the guinea pig model. J. Infect. Dis. 172:1212-1220. [DOI] [PubMed] [Google Scholar]
- 23.Hayes, K., and H. Gibas. 1971. Placental cytomegalovirus infection without fetal involvement following primary infection in pregnancy. J. Pediatr. 79:401-405. [DOI] [PubMed] [Google Scholar]
- 24.Hostetler, K. Y. 2009. Alkoxyalkyl prodrugs of acyclic nucleoside phosphonates enhance oral antiviral activity and reduce toxicity: current state of the art. Antiviral Res. 82:A84-A98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ivarsson, S. A., B. Lernmark, and L. Svanberg. 1997. Ten-year clinical, developmental, and intellectual follow-up of children with congenital cytomegalovirus infection without neurologic symptoms at one year of age. Pediatrics 99:800-803. [DOI] [PubMed] [Google Scholar]
- 26.Jacquemard, F., M. Yamamoto, J. M. Costa, S. Romand, E. Jaqz-Aigrain, A. Dejean, F. Daffos, and Y. Ville. 2007. Maternal administration of valaciclovir in symptomatic intrauterine cytomegalovirus infection. BJOG 114:1113-1121. [DOI] [PubMed] [Google Scholar]
- 27.Kern, E. R., D. J. Collins, W. B. Wan, J. R. Beadle, K. Y. Hostetler, and D. C. Quenelle. 2004. Oral treatment of murine cytomegalovirus infections with ether lipid esters of cidofovir. Antimicrob. Agents Chemother. 48:3516-3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kern, E. R., C. Hartline, E. Harden, K. Keith, N. Rodriguez, J. R. Beadle, and K. Y. Hostetler. 2002. Enhanced inhibition of orthopoxvirus replication in vitro by alkoxyalkyl esters of cidofovir and cyclic cidofovir. Antimicrob. Agents Chemother. 46:991-995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kimberlin, D. W., E. P. Acosta, P. J. Sanchez, S. Sood, V. Agrawal, J. Homans, R. F. Jacobs, D. Lang, J. R. Romero, J. Griffin, G. A. Cloud, F. D. Lakeman, and R. J. Whitley. 2008. Pharmacokinetic and pharmacodynamic assessment of oral valganciclovir in the treatment of symptomatic congenital cytomegalovirus disease. J. Infect. Dis. 197:836-845. [DOI] [PubMed] [Google Scholar]
- 30.Kimberlin, D. W., C. Y. Lin, P. J. Sanchez, G. J. Demmler, W. Dankner, M. Shelton, R. F. Jacobs, W. Vaudry, R. F. Pass, J. M. Kiell, S. J. Soong, and R. J. Whitley. 2003. Effect of ganciclovir therapy on hearing in symptomatic congenital cytomegalovirus disease involving the central nervous system: a randomized, controlled trial. J. Pediatr. 143:16-25. [DOI] [PubMed] [Google Scholar]
- 31.Kumar, M. L., and G. A. Nankervis. 1978. Experimental congenital infection with cytomegalovirus: a guinea pig model. J. Infect. Dis. 138:650-654. [DOI] [PubMed] [Google Scholar]
- 32.Lombardi, G., F. Garofoli, P. Villani, M. Tizzoni, M. Angelini, M. Cusato, L. Bollani, A. De Silvestri, M. Regazzi, and M. Stronati. 2009. Oral valganciclovir treatment in newborns with symptomatic congenital cytomegalovirus infection. Eur. J. Clin. Microbiol. Infect. Dis. 28:1465-1470. [DOI] [PubMed] [Google Scholar]
- 33.Marshall, B. C., and W. C. Koch. 2009. Antivirals for cytomegalovirus infection in neonates and infants: focus on pharmacokinetics, formulations, dosing, and adverse events. Paediatr. Drugs 11:309-321. [DOI] [PubMed] [Google Scholar]
- 34.Meine Jansen, C. F., M. C. Toet, C. M. Rademaker, T. F. Ververs, L. J. Gerards, and A. M. van Loon. 2005. Treatment of symptomatic congenital cytomegalovirus infection with valganciclovir. J. Perinat. Med. 33:364-366. [DOI] [PubMed] [Google Scholar]
- 35.Muller, A., A. M. Eis-Hubinger, G. Brandhorst, A. Heep, P. Bartmann, and A. R. Franz. 2008. Oral valganciclovir for symptomatic congenital cytomegalovirus infection in an extremely low birth weight infant. J. Perinatol. 28:74-76. [DOI] [PubMed] [Google Scholar]
- 36.Nassetta, L., D. Kimberlin, and R. Whitley. 2009. Treatment of congenital cytomegalovirus infection: implications for future therapeutic strategies. J. Antimicrob. Chemother. 63:862-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nelson, C. T., and G. J. Demmler. 1997. Cytomegalovirus infection in the pregnant mother, fetus, and newborn infant. Clin. Perinatol. 24:151-160. [PubMed] [Google Scholar]
- 38.Nigro, G., S. P. Adler, R. La Torre, and A. M. Best. 2005. Passive immunization during pregnancy for congenital cytomegalovirus infection. N. Engl. J. Med. 353:1350-1362. [DOI] [PubMed] [Google Scholar]
- 39.Oliver, S. E., G. A. Cloud, P. J. Sanchez, G. J. Demmler, W. Dankner, M. Shelton, R. F. Jacobs, W. Vaudry, R. F. Pass, S. J. Soong, R. J. Whitley, and D. W. Kimberlin. 2009. Neurodevelopmental outcomes following ganciclovir therapy in symptomatic congenital cytomegalovirus infections involving the central nervous system. J. Clin. Virol. 46(Suppl. 4):S22-S26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parker, S., A. M. Siddiqui, C. Oberle, E. Hembrador, R. Lanier, G. Painter, A. Robertson, and R. M. Buller. 2009. Mousepox in the C57BL/6 strain provides an improved model for evaluating anti-poxvirus therapies. Virology 385:11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pass, R. F. 2001. Cytomegalovirus, p. 2675-2705. In D. M. Knipe, P. M. Howley, D. E. Griffin, et al. (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 42.Pass, R. F., S. Stagno, G. J. Myers, and C. A. Alford. 1980. Outcome of symptomatic congenital cytomegalovirus infection: results of long-term longitudinal follow-up. Pediatrics 66:758-762. [PubMed] [Google Scholar]
- 43.Pass, R. F., C. Zhang, A. Evans, T. Simpson, W. Andrews, M. L. Huang, L. Corey, J. Hill, E. Davis, C. Flanigan, and G. Cloud. 2009. Vaccine prevention of maternal cytomegalovirus infection. N. Engl. J. Med. 360:1191-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Puliyanda, D. P., N. S. Silverman, D. Lehman, A. Vo, S. Bunnapradist, R. K. Radha, M. Toyoda, and S. C. Jordan. 2005. Successful use of oral ganciclovir for the treatment of intrauterine cytomegalovirus infection in a renal allograft recipient. Transpl. Infect. Dis. 7:71-74. [DOI] [PubMed] [Google Scholar]
- 45.Quenelle, D., M. Prichard, E. Harden, D. Collins, T. L. Rice, G. Painter, A. Robertson, and E. Kern. 2009. Comparative efficacy of treatment with CMX001 versus acyclovir in BALB/c mince infected with herpes simplex virus. Antiviral Res. 82:A51-A82. [Google Scholar]
- 46.Quenelle, D. C., M. N. Prichard, K. A. Keith, D. E. Hruby, R. Jordan, G. R. Painter, A. Robertson, and E. R. Kern. 2007. Synergistic efficacy of the combination of ST-246 with CMX001 against orthopoxviruses. Antimicrob. Agents Chemother. 51:4118-4124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Toth, K., J. F. Spencer, D. Dhar, J. E. Sagartz, R. M. Buller, G. R. Painter, and W. S. Wold. 2008. Hexadecyloxypropyl-cidofovir, CMX001, prevents adenovirus-induced mortality in a permissive, immunosuppressed animal model. Proc. Natl. Acad. Sci. U. S. A. 105:7293-7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wan, W. B., J. R. Beadle, C. Hartline, E. R. Kern, S. L. Ciesla, N. Valiaeva, and K. Y. Hostetler. 2005. Comparison of the antiviral activities of alkoxyalkyl and alkyl esters of cidofovir against human and murine cytomegalovirus replication in vitro. Antimicrob. Agents Chemother. 49:656-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang, H., and R. Datema. 1991. Prolonged and potent therapeutic and prophylactic effects of (S)-1-[(3-hydroxy-2-phosphonylmethoxy)propyl]cytosine against herpes simplex virus type 2 infections in mice. Antimicrob. Agents Chemother. 35:1596-1600. [DOI] [PMC free article] [PubMed] [Google Scholar]