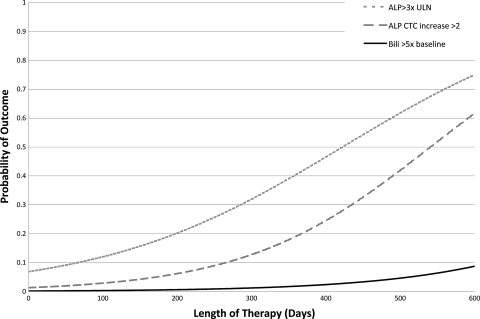
FIG. 4.
Hepatic outcomes by length of therapy. An ALP CTC increase of >2 controlled for the FDA dosing group, a bilirubin level >5× the baseline controlled for FDA dosing group and initial bilirubin concentration, and an ALP level >3× the ULN controlled for FDA dosing group, initial ALP concentration, and mg/kg dose.