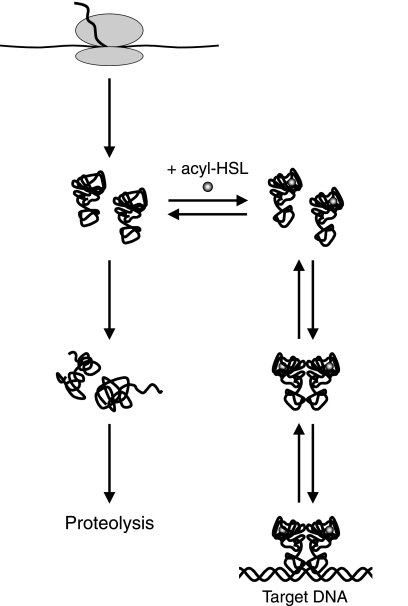
FIG. 7.
General model for acyl-HSL receptor states in vivo. Nascent polypeptides fold into a functional but relatively unstable state. In the absence of an appropriate acyl-HSL, the polypeptides refold into nonfunctional conformations, which aggregate or are targeted for proteolysis. The folded polypeptides exist as monomers at sufficiently low concentrations, but as the acyl-HSL-bound monomers accumulate, they form homodimers capable of high-affinity binding to target promoters. The concentration of monomers required for dimer formation varies among different QscR homologs.