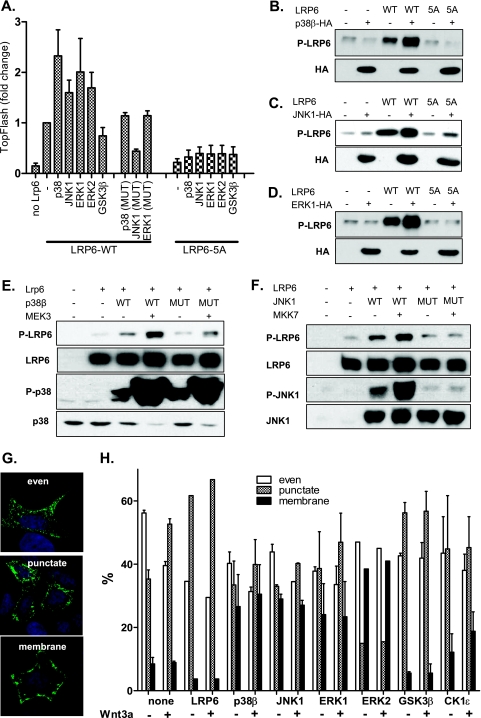
FIG. 4.
MAPKs promote LRP6 phosphorylation and WNT/β-catenin signaling in cells. (A) HEK293 cells were transfected with plasmids encoding full-length LRP6 (LRP6WT) or LRP6 mutants with all PPPS/TP sites mutated to PPPAP (LRP6-5A) in combination with indicated MAPKs. Synergistic effects of MAPKs (but not their mutated variants, p38β-T106M, ERK1-K72R, and JNK1-T183A/Y185F) and LRP6WT, but not MAPKs and LRP6-5A on TCF/LEF-dependent transcription were found using the TopFlash reporter system. (B and C) Cells were transfected as described for panel A with p38β (B), JNK1 (C), and ERK1 (D), and the phosphorylation of LRP6 was determined by Western blotting. Presence of overexpressed kinases is indicated by probing against the HA tag. (E and F) HEK293 cells were transfected as indicated (mutated MAPKs were identical to those described for panel A), and the phosphorylation of LRP6 at Ser1490 was determined by Western blotting. The activities of MAPKKs (MEK3 and MKK7) were confirmed by probing against activated forms of p38 (phospho-Thr180/Tyr182) and JNK1 (phospho-Thr183/Tyr185). (G) HEK293 cells were transfected with AU-axin1 together with the plasmids encoding proteins as indicated. The cells were either stimulated or not with rmWNT-3A. Cells were fixed and stained for axin, and based on the axin distribution, they were sorted into the following three categories: even, punctate, and membranous. Axin distribution under each condition was determined by counting of at least 200 cells in three independent replicates and is shown as mean ± SEM (H). Blue, DAPI; green, axin-GFP.