FIG. 2.
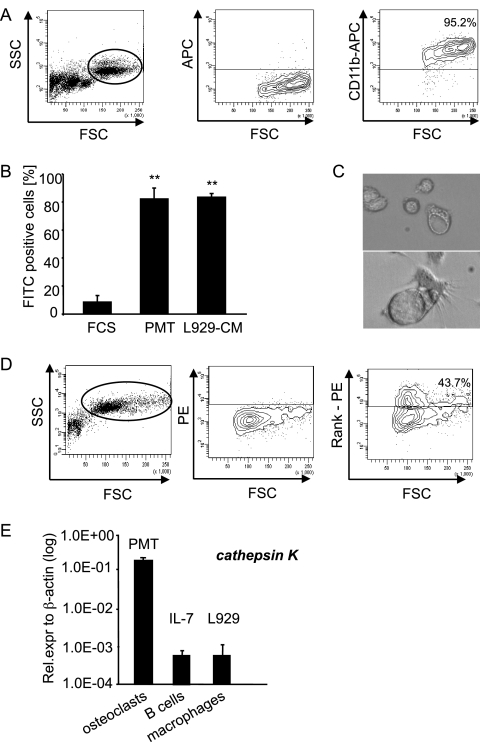
PMT-derived macrophages phagocytose and have the potential to differentiate into osteoclasts. (A) Characterization of macrophages. PMTwt-stimulated (6 days, 6.5 nM) bone marrow cells were stained for the macrophage marker CD11b. The right panel shows staining of the gated macrophage population. (B) Functional characterization of the macrophage population. Bone marrow cells stimulated for 6 days with PMTwt and untreated bone marrow cells were incubated with FITC-labeled latex beads at 37°C or at 4°C (negative control). As a positive control macrophages were used that had been generated by selection of bone marrow cells with L929 conditioned medium. The CD11b-containing population was analyzed for FITC-positive cells. The diagram shows the percentage of FITC positive cells minus values of negative controls. The indicated standard deviation was calculated from three independent experiments (mean ± SD; n = 3). The statistical significance was assessed by using a paired Student t test (**, P < 0.01). (C) Characterization of osteoclasts. A mixed preparation of murine bone marrow cells was incubated with 6.5 nM PMTwt for 14 days. Osteoclasts were identified by their typical morphology. (D) RANK expression. The formation of osteoclasts was analyzed by FACS using the osteoclast specific surface marker RANK. The right panel shows the staining of the gated population in the left panel, and the middle panel shows unstained control cells. (E) Transcription level of the osteoclastic marker cathepsin K. The amounts of cathepsin K after PMTwt treatment (6.5 nM, 12 days), IL-7-generated B cells (10 ng/ml, 5 days), and L929-generated macrophages (30% L929-CM, 6 days) were analyzed by quantitative RT-PCR using cathepsin K-specific primers and normalization to actin expression. The indicated SD was calculated from two independent experiments (mean ± SD; n = 2).