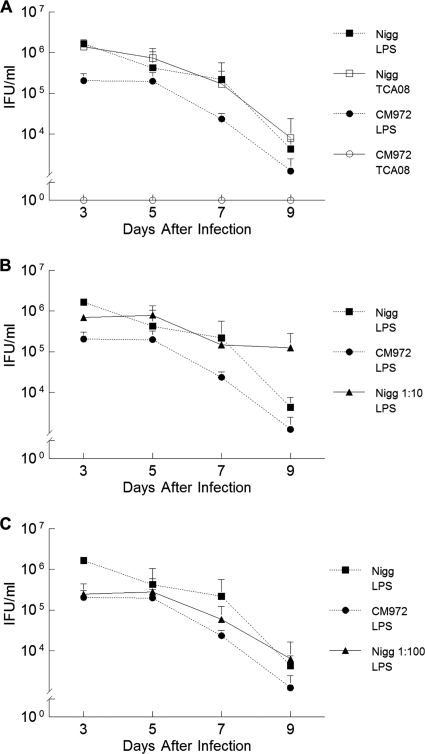
FIG. 4.
Cervicovaginal shedding of chlamydiae by mice infected with C. muridarum Nigg and CM972. (A) Groups of mice were infected with either Nigg or CM972, and cervicovaginal shedding over subsequent days was assessed by immunofluorescent staining using a mouse monoclonal antibody directed against chlamydial LPS (Nigg plus CM972) or a rabbit polyclonal antibody directed against pMoPn-encoded TCA08. Immunofluorescent staining with an anti-TCA08 antibody successfully discriminates between C. muridarum Nigg (open squares) and plasmid-deficient CM972 (open circles at 100) shed by infected mice. CM972 infection indicated by anti-LPS staining (closed circles) was less infectious than Nigg (closed squares) (P = 0.03 by two-way RM ANOVA). (B) Cervicovaginal shedding of chlamydiae by mice infected with C. muridarum Nigg and CM972 at ratio of 1:10. The total bacterial burden was detected by immunofluorescent staining with anti-LPS antibody. Results of shedding by parallel groups infected with Nigg or CM972 alone are indicated as controls. By day 3 of infection, mice infected with the mixed inoculum were shedding more chlamydiae than mice infected solely with CM972 (P = 0.04; two-way RM ANOVA with multiple comparisons), indicating that Nigg had quickly become predominant. (C) Cervicovaginal shedding of chlamydiae by mice infected with C. muridarum Nigg and CM972 at ratio of 1:100. The total bacterial burden was detected by immunofluorescent staining with anti-LPS antibody. Results of shedding by parallel groups infected with Nigg or CM972 alone are indicated as controls. Mice infected with 103 Nigg plus 105 CM972 shed chlamydiae in amounts similar to those seen with mice infected with wild-type Nigg by day 5 of infection, and levels were equivalent to those seen with mice infected with Nigg alone by day 9. Data represent the means ± standard deviations of the results for each group of 5 mice, and the experiment was performed twice.