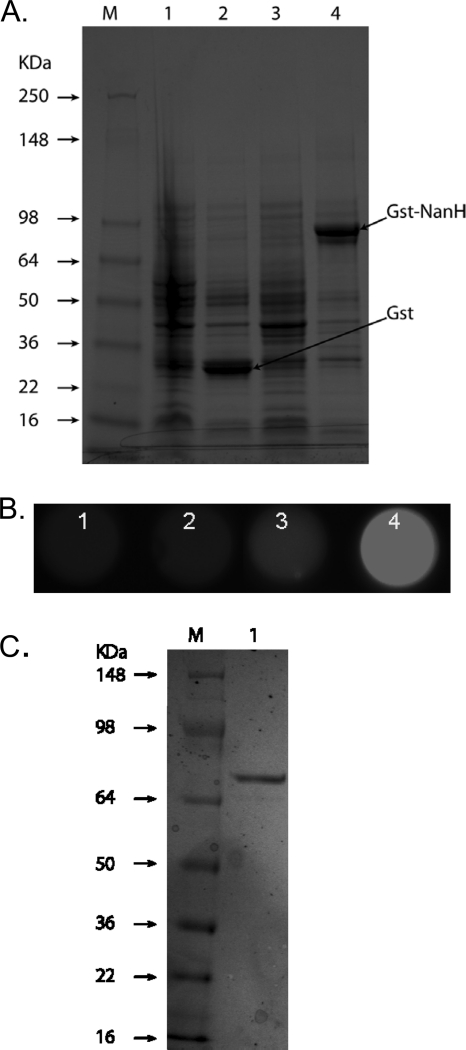
FIG. 2.
Expression of T. forsythia NanH in E. coli as a GST fusion protein. (A). SDS-PAGE of E. coli clones harboring the control (BL21/pGEX-4T; lanes 1 and 2) or recombinant (BL21/pGEX-nanH) plasmid in the presence (lanes 2 and 4) or absence (lanes 1 and 3) of IPTG. E. coli cultures grown to an absorbance of 0.2 at 600 nm were incubated for an additional 3 h in the presence or absence of IPTG. Cell lysates (50 μg protein for each) were separated on 10% gels and stained with Coomassie. (B) Sialidase activity assayed in lysates of corresponding clones in microtiter wells (BL21/pGEX-4T, lanes 1 [uninduced] and 2 [induced]; BL21/pGEX-nanH, lanes 3 [uninduced] and 4 [induced]; 10 μg protein lysate for each) using 4-MU-NeuAc as a substrate. Fluorescence was visualized on a UV transilluminator. (C) SDS-PAGE of purified rGST-NanH protein on a 4-to-12%-gradient polyacrylamide gel. Lane M, protein markers; lane 1, rGst-NanH.