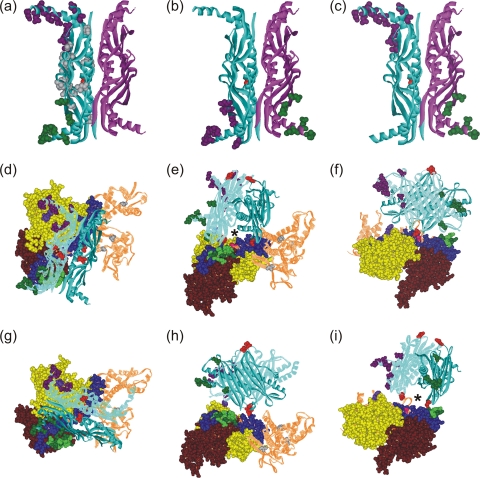
FIG. 5.
Models of the orientation of SecB and SecA within a complex. SecB residues that cross-link to SecA shown as CPK models colored according to the cross-linking partner on SecA: purple for SecAV9 and G11, green for residues on SecA between 600 and 609. (a) SecB with both protomers involved in contact on the same dimer; (b and c) each of the dimers contains one protomer involved in contacts, directly across the dimer interface (b) or diagonally opposite (c). Two orientations that satisfy all cross-links are shown: one in panels d to f and a second in panels g to i. The SecA protomer has the subdomains that cross-link to SecB in CPK representation: nucleotide binding folds NBF1 (yellow) and NBF2 (light brown), the precursor binding domain (PBD; pink), the helical scaffold domain (HSD; blue), and the linker helix (green). The remaining domains that show no contacts, the helical wing domain and the intramolecular regulator, are shown as brown lined ribbons. The residues that show cross-links are purple (V9 and G11), light green (601, 603, 605, 607, and 609), and red (636). A second protomer could be positioned on the upper surface through symmetrically related residues on the protomers of SecB. The red CPK residues (SecB35) are the sites that contact the long blue helix at residue SecA636 (red CPK). An asterisk marks the channel that would be occupied by a precursor polypeptide as described in the text.