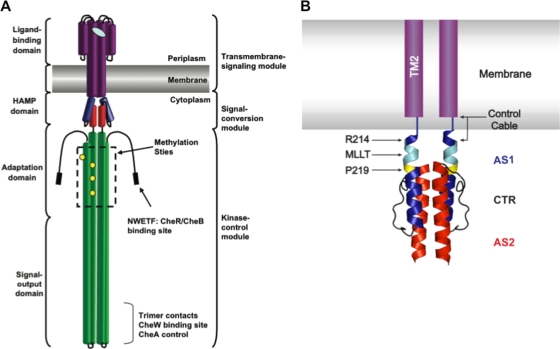
FIG. 1.
Domain architecture of the aspartate chemoreceptor. (A) The cartoon, based on a figure from Hazelbauer et al. (2008) (19), illustrates the architecture of the aspartate chemoreceptor. Protein structural domains are labeled on the left, and functional modules are labeled on the right. (B) Schematic of TM2 and the control cable region attached to a ribbon diagram of the solution NMR structure of the Af1503 HAMP domain four-helix bundle (22). TM2 is shown in purple within the membrane. The control cable of TarEc consists of 5 amino acyl residues (Gly-Ile-Arg-Arg-Met) that connect TM2 and AS1 of HAMP. AS1 is shown in blue, AS2 is shown in red, and the 14-residue AS1-AS2 connector (CTR) is shown in black. The residue equivalent to Arg-214 in TarEc is also highlighted in blue, the conserved Pro residue (Pro-219 in TarEc) is highlighted in yellow, and residues equivalent to the MLLT sequence between TM2 and Pro-219 in TarEc are highlighted in cyan.