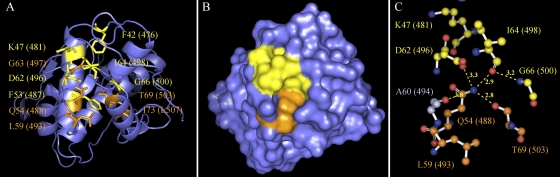
FIG. 3.
Mapping of a highly conserved pocket in the crystal structure of hydrolase metallo (Zn) DD-peptidase. The six prokaryote-specific residues F42, K47, F53, D62, I64, and G66 (yellow) and five commonly conserved residues Q54, G63, L59, T69, and I73 (orange) are mapped on the structure of hydrolase metallo (Zn) DD-peptidase (PDB entry 1LBU). The corresponding residues in ExeA are indicated in parentheses. (A) Cartoon view of the structure with the above residues illustrated as sticks. (B) Surface view of the structure. (C) Composition of the putative peptidoglycan binding pocket. Nitrogen atoms and oxygen atoms are marked in blue and red, respectively. The possible hydrogen bonds involved in the composition of the pocket are illustrated with the distances indicated in Å. F42, F53, G63, and I73 are buried beneath the pocket and are not shown in panel C. The images were generated by PyMOL Molecular Graphics System, version 1.3 (Schrödinger, LLC).