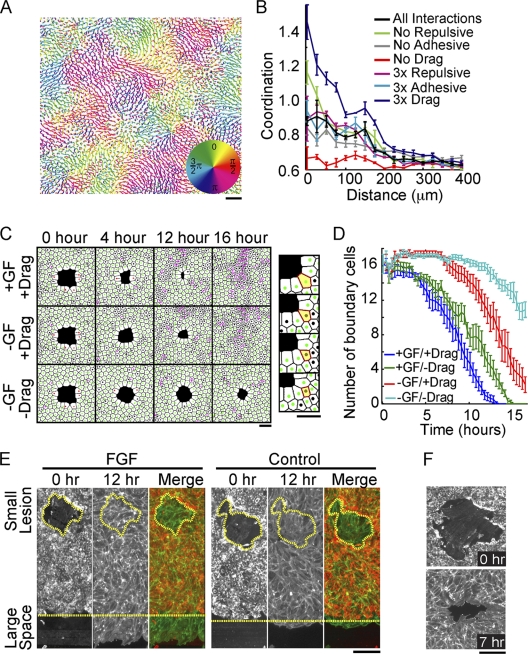
FIG. 4.
Drag mediates coordinated migration and small-lesion closure even in the absence of directed motility. (A) Cell tracks from a computational model run for 16 simulated hours colored according to the direction of migration. (B) Quantification of coordinated migration in simulated tracks with indicated relative steering changes (for details, see the text). (C) Images of cell monolayers derived from the computational model representing 0, 4, 12, and 16 simulated hours after the introduction of a small lesion. Steering terms were included (+) or eliminated (−) as indicated. (Right) Zoomed image of a simulated monolayer with an excluded boundary cell highlighted in yellow. (D) Number of boundary cells surrounding a small lesion plotted as a function of time for different steering conditions, suggesting that drag helps to reduce the number of cells bordering small lesions. All measurements represent the averages of three replicates, with error bars indicating standard errors. (E) Differential migration into small and large open spaces. Cells were assayed as in Fig. 1D in the presence (left) and absence (right) of FGF. Merge, merged image of the monolayer before (red) and after (green) a 12-hour migration period, showing that small lesions can close in the absence of directed migration. The perimeter of the small wound and edge of the sheet boundary are marked with yellow dashed lines. (F) Phalloidin staining for polymerized actin in endothelial cells surrounding a small lesion showing no apparent actomyosin ring, supporting a migration- over a constriction-based closure mechanism. Bars, 150 μm.