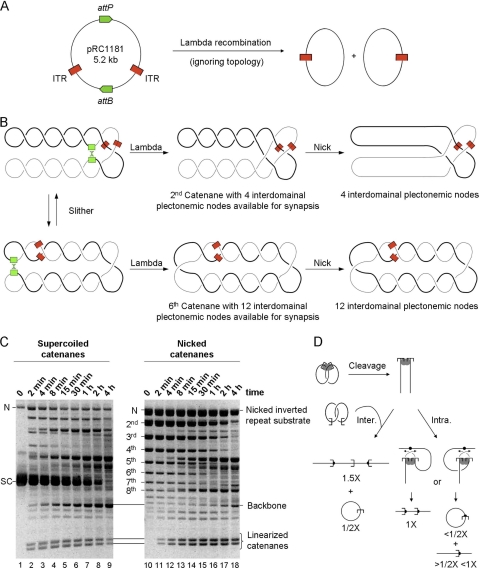
FIG. 5.
DNA intertwining accelerates transposition. (A) An illustration of the phage lambda integrase reaction. Recombination between the phage lambda attP and attB sites separates the transposon ends onto two circles of almost identical size. Topological features of the reaction have been omitted but are shown in panel B. (B) An illustration of how phage lambda recombination can be used to generate catenated single-end transposition substrates. The lambda attP and attB sites (green) divide the plasmid into two equal-sized segments, indicated by the thick and thin lines. The att sites meet by random collision, trapping a number of plectonemic nodes. These are converted to catenane nodes by lambda recombination. The number of trapped nodes converted ranges from four, the minimum allowed by the mechanism of recombination, up to the maximum number of nodes present in the substrate. Lambda recombination preserves any supercoiling nodes that are not converted to catenane nodes (see the top element of the central panel). After recombination, each of the catenated circles contains a single transposon end (red). The catenated circles can slither past each other, providing opportunities for the transposon ends to meet in the right-handed crossing points. Treatment with the Nb.BsrDI endonuclease introduces a nick into each of the catenated circles, releasing any supercoiling that is present (rightmost panel). (C) The kinetics of transposition reactions with supercoiled and nicked catenane substrates. The absence of supercoiling in the nicked substrate reveals the distribution of the catenanes. The second catenane, with four nodes, is the most abundant product of lambda recombination. Its identity was confirmed in a high-resolution gel (not shown, but see reference 26). About 65% of the substrate was recombined by the lambda integrase, as revealed by the abundance of the unrecombined nicked inverted-repeat substrate in the rightmost panel (N). The backbone fragment produced from the 35% of the inverted-repeat substrate that remains in these reactions provides a useful internal control for the kinetic analysis (see the text for details). A photograph of an ethidium bromide-stained agarose gel is shown. (D) Products of transposition with catenated single-end substrates. Cleavage at the transposon ends yields two linear products of similar sizes. Intermolecular integration into an unreacted substrate plasmid, which is the only target available in these reactions, releases one of the half-circles and generates a linear product 1.5 times as large as the original plasmid used to generate the catenanes (i.e., 1.5X). Intramolecular events produce two alternative outcomes depending on orientation of the target site: a linear product the size of the original plasmid (1X) or a pair of products, namely, an open circle plus a linear product that together equal the size of the plasmid.