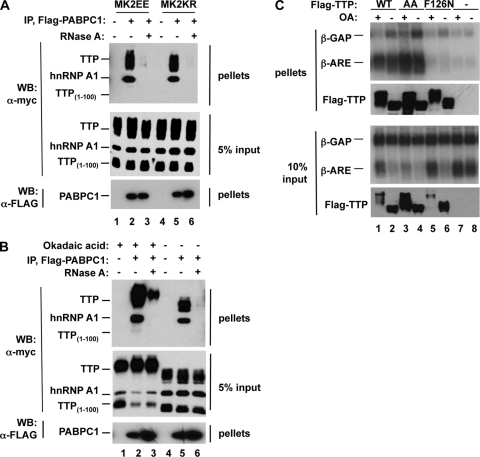
FIG. 5.
Phosphorylation of TTP does not impair mRNA binding. (A) Coimmunoprecipitation assays performed with extracts derived from HEK293T cells coexpressing FLAG-tagged cytoplasmic poly(A)-binding protein (PABPC1) (lanes 2, 3, 5, and 6) together with Myc-tagged TTP, hnRNP A1, TTP1-100 (lanes 1 to 6), and MK2EE (lanes 1 to 3) or MK2KR (lane 4 to 6). Immunoprecipitations (IP) were performed in the absence (lanes 1, 2, 4, and 5) or presence (lanes 3 and 6) of RNase A. Lanes 1 and 4 are negative controls from cells expressing no FLAG-tagged protein. Myc-tagged hnRNP A1 served as a positive RNA binding control, whereas the N-terminal domain of TTP (TTP1-100) served as a negative control that does not bind RNA. Pellet and 5% of input fractions were loaded as indicated on the right and subjected to immunoblotting (Western blotting [WB]) with anti-myc or anti-FLAG antibodies as indicated on the left. (B) Coimmunoprecipitation assays as in panel A, except that cells were pretreated with the PP2A inhibitor okadaic acid (OA; lanes 1 to 3) or the vehicle (lanes 4 to 6) 2 h prior to lysate preparation. (C) Northern blot assays of RNA immunoprecipitated from cells coexpressing β-GAP and β-ARE reporter mRNAs together with FLAG-tagged wild-type (WT) TTP (lanes 1 and 2), or mutant versions of TTP with serines 52 and 178 mutated to alanines (AA, lanes 3 and 4) or bearing a point mutation that abolishes RNA binding (F126N, lanes 5 and 6). Prior to immunoprecipitation, cells were treated for 2 h with (+) or without (−) okadaic acid. TTP protein levels are shown in Western blot assays below each Northern blot assay. Upper panels, pellets; lower panels, 10% input. Lanes 7 and 8, immunoprecipitates from cells expressing no exogenous TTP protein.