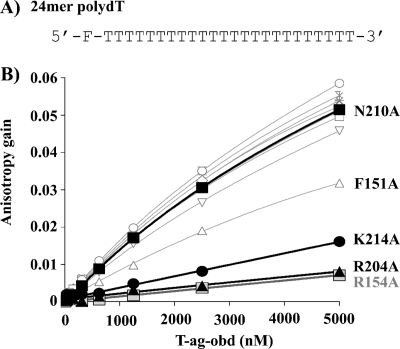
FIG. 4.
Binding of T-Ag OBD proteins to poly(dT) DNA measured by fluorescence anisotropy. (A) Sequence of the 24-nucleotide poly(dT) probe used in this study. (B) Measurement of the T-Ag OBD-poly(dT) interaction via fluorescent anisotropy. Binding isotherms were measured in triplicate with 15 nM single-stranded DNA probe and increasing concentrations of the wild-type T-Ag-OBD (○) or of the V150A (□), F151A (Δ), S152A (▿), R154A ( ), L156A (+), T199A (⋄), H201A (*), H203A (×), R204A (▴), N210A (▪), or K214A (•) mutant protein. Curves were obtained by a nonlinear regression and fitting to a one-binding-site equilibrium. Error bars are not visible on the graph as they are smaller than the size of the symbols. Kds (dissociation constants) were not calculated for these binding curves because they are not saturated although the relative affinities may be compared.
), L156A (+), T199A (⋄), H201A (*), H203A (×), R204A (▴), N210A (▪), or K214A (•) mutant protein. Curves were obtained by a nonlinear regression and fitting to a one-binding-site equilibrium. Error bars are not visible on the graph as they are smaller than the size of the symbols. Kds (dissociation constants) were not calculated for these binding curves because they are not saturated although the relative affinities may be compared.