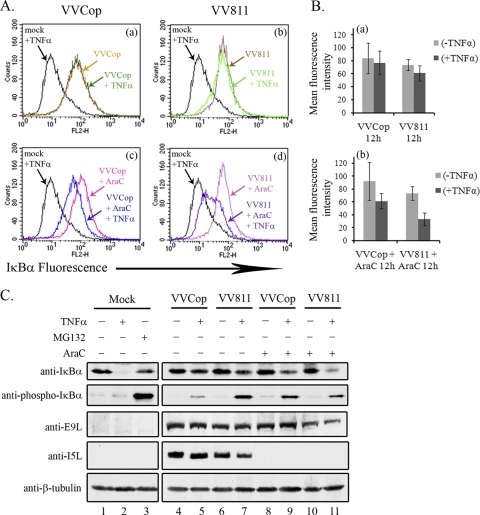
FIG. 9.
A late vaccinia virus protein regulates IκBα degradation. (A) HeLa cells were infected with VVCop or VV811 at an MOI of 5 for 12 h, and cells were either mock treated or treated with TNF-α for 20 min. Cells were treated with 80 μg/ml of araC to inhibit late poxvirus gene expression. Mock-infected cells treated with MG132 and TNF-α were used as a positive control for inhibition of IκBα degradation. IκBα levels were detected by flow cytometry by staining with mouse anti-IκBα, followed by fluorescently tagged anti-mouse secondary antibody, and flow cytometry analysis was performed to examine IκBα fluorescence. (B) Quantification of IκBα fluorescence was determined from the mean fluorescence intensities represented by the average from three independent experiments. Error bars represent standard deviations. (C) HeLa cells were mock infected or infected with VVCop or VV811 for 12 h at an MOI of 5 and either mock treated or treated with TNF-α for 20 min. Additionally, cells infected with VVCop or VV811 were treated with 80 μg/ml araC, to inhibit late genes at 12 h postinfection. MG132 combined with TNF-α treatment was used as a positive control for inhibition of IκBα degradation. Cytoplasmic extracts were generated and Western blotted with antibodies to IκBα, phospho-IκBα, E9L, I5L, and β-tubulin, as a loading control.