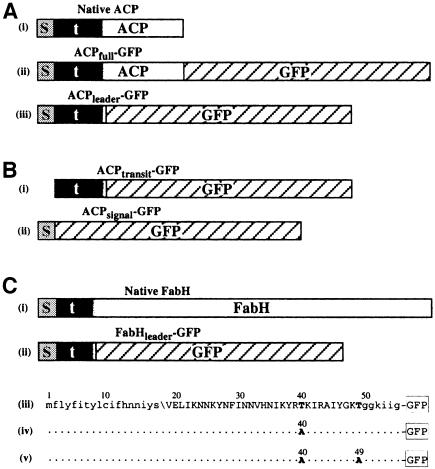
Fig. 1. Schematics of GFP fusions expressed in P.falciparum. (A) Plasmodium falciparum ACP with (i) bipartite leader, and GFP fusions (ii) ACPfull–GPF and (iii) ACPleader–GFP (s, signal peptide; t, transit peptide; shaded transition between the transit peptide and ACP indicates uncertainty of the precise boundary). (B) Signal and transit peptide deletion GFP fusions (i) ACPtransit–GFP and (ii) ACPsignal–GFP. (C) Plasmodium falciparum FabH protein with (i) bipartite leader and (ii) GFP fusion FabHleader–GFP represented schematically and (iii) in sequence form. Mutations engineered into fusions FabHleader[T40A]–GFP and FabHleader[T40/49A]–GFP are indicated in (iv) and (v), respectively. N-terminal lower case sequence represents the signal peptide with the predicted cleavage point indicated (\). Upper case sequence indicates transit peptide domain and the C-terminal lower case sequence indicates the first conserved residues shared amongst FabH sequences in other organisms.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.