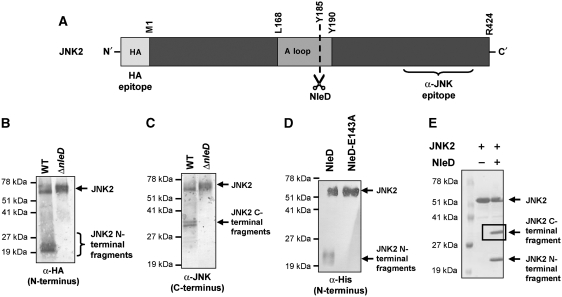
Figure 2.
NleD clips JNK within the activation loop. (A) Schematic diagram of JNK2. The location of the HA and anti-JNK antibody epitopes, activation loop (A loop), T and Y phosphorylation sites and NleD-cleavage site are indicated. (B, C) NleD cleaves JNK2 in vivo. HeLa cells were transfected with plasmids expressing HA-tagged JNK2. At 25 h post-transfection, the cells were infected with wild-type (WT) or nleD deletion mutant (ΔnleD) EPEC. After 2.5 h, proteins were extracted from the infected HeLa cells, immunoprecipitated using anti-HA antibody and subjected to western blot analysis using anti-HA (B) or anti-JNK (C) antibodies. JNK2 and its degradation fragments are indicated. Negative control using non-transfected cells confirmed the antibody specificity (data not shown). (D, E) NleD cleaves JNK2 in vitro. Purified, N-terminally tagged, 6His-JNK2 was incubated with purified NleD or NleD-E143A. After 60 min, the reaction was stopped using SDS loading buffer. To estimate the size of the N-terminal fragment of the clipped JNK2, the reaction mixture was subjected to western blot analysis using anti-6His antibody (D). Intact JNK2 and its N-terminal fragments are indicated. To determine the size of the two JNK2 fragments, the reaction mixture was analysed also by SDS–PAGE followed by Commassie blue staining (E). JNK2 and its degradation fragments are indicated. The framed C-terminal band was subjected to N-terminal sequencing analysis.