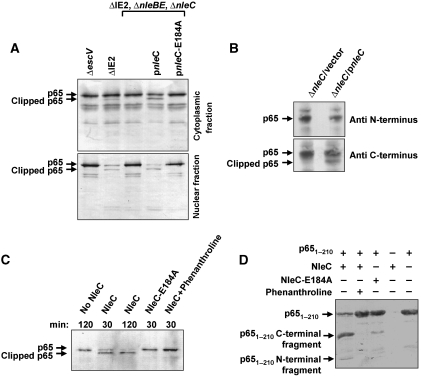
Figure 5.
NleC induces p65 cleavage, which is associated with reduced nuclear p65 levels. (A) NleC induces p65 cleavage in vivo. HeLa cells were infected for 3 h with the ΔIE2 ΔnleBE, ΔnleC mutant EPEC that was complemented, or not, with plasmids expressing NleC or mutated NleC (NleC-E184A), as indicated. Proteins were extracted from the infected HeLa cells, separated into cytosolic and nuclear fractions and subjected to western blot analysis using anti-p65 antibody. An EPEC mutant deficient in TTSS activity (ΔescV) and EPEC deleted of the IE2 region (ΔIE2) served as negative and positive controls, respectively; the latter was used here as wild type. Intact and clipped p65 are indicated. (B) NleC induces clipping the N-terminal end of p65. HeLa cells were infected for 3 h with the ΔnleC mutant EPEC that was complemented with plasmids expressing NleC or vector only, as indicated. Proteins were extracted from the infected HeLa cells and subjected to western blot analysis using anti-N-terminus of p65 or anti-C-terminus of p65 antibodies. Intact and clipped p65 are indicated. (C) NleC induced p65 cleavage in vitro. The cytosolic fraction of HeLa extracts was combined with NleC, NleC-E184A, or buffer alone in the presence or absence of phenanthroline, a Zn metalloprotease inhibitor, as indicated. The incubation time is indicated above each lane. Reaction products were visualized by western blot analysis using anti-p65 antibody. Intact and clipped p65 are indicated. (D) NleC directly cuts p651–210. Purified p651–210 and NleC were incubated in a reaction mixture at a molar ratio of 40:1, in the presence or absence of phenanthroline. The reaction was stopped by addition of SDS loading buffer and proteins separated by SDS–PAGE and visualized by Coomassie blue staining. p651–210 and its degradation fragments are indicated. NleC does not appear in this gel.