Figure 1.
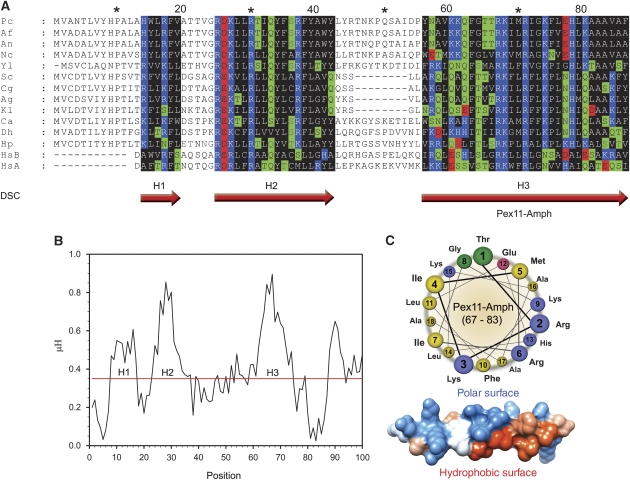
Pex11 contains a conserved N-terminal amphipathic helix. (A) Sequence alignment of N-terminal regions of Pex11 proteins from various species. Putative α-helices were predicted using the DSC programme and are marked with red arrows (H1–H3). Residues in predicted α-helices are coloured based on the physico-chemical properties of amino acids as follows: hydrophilic, charged: D, E (red), K, R, H (blue); hydrophilic, neutral: S, T, Q, N (green); hydrophobic: A, V, L, I, M, W, F, Y, G, P (black). The conserved helix H3 consists of hydrophobic and polar, positively charged residues arranged in a recurrent manner. Abbreviations and accessions numbers used in sequence alignments: Pc—Penicillium chrysogenum, AAQ08763; Af—Aspergillus fumigatus, EAL88627; An—Aspergillus nidulans, EAA65086; Nc—Neurospora crassa, XP_960428; Yl—Yarrowia lipolytica, CAG81724; Sc—Saccharomyces cerevisae, CAA99168; Cg—Candida glabrata, CAG58440; Ag—Ashbya gossypii, AAS54890; Kl—Kluyveromyces lactis, CAG99119; Ca—Candida albicans, EAK92906; Db—Debaryomyces hansenii, CAG84534; Hp—Hansenula polymorpha, DQ645582; HsA—Homo sapiens Pex11α, AAH09697; HsB—Homo sapiens Pex11β, AAH11963. Asterisk and numbers mark amino acids positions in the alignment. (B) Plot of hydrophobic moments (μH) for the N-terminus of PcPex11. The positions of predicted α-helices are marked with H1–H3. The highest value of the μH was obtained for the long putative helix H3, suggesting strong amphipathic properties for this motif. (C) Helical wheel representation and 3D model of the portion of the putative helix H3 with the strongest amphipathic properties, tentatively termed Pex11-Amph (PcPex11 amino acids 67–83). In the helical wheel representation, the amino acids are coloured according to the physico-chemical properties of the side chains (hydrophobic—yellow; polar, positively charged—blue; polar, negatively charged—pink; polar, uncharged—green). In the 3D model of an ideal α-helix built on the basis of the Pex11-Amph sequence, the computed surfaces are marked as follows: hydrophobic (red), positively charged (blue), other amino acids (white).