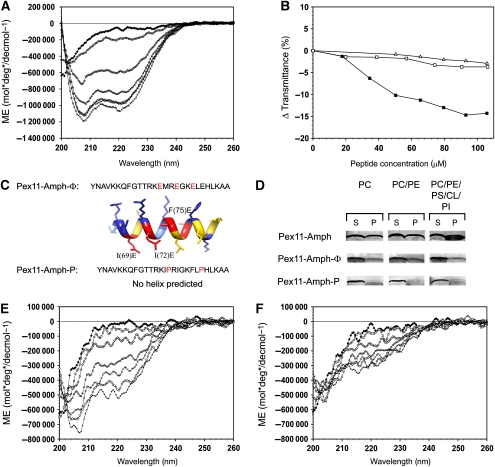
Figure 2.
Interaction of the Pex11-Amph with model membranes. (A) The secondary structure of the Pex11-Amph peptide was analysed using CD spectroscopy. The spectrum shows that this peptide is unstructured in phosphate buffer (♦). Addition of increasing amounts of 2,2,2-trifluoroethanol (TFE) induces changes in the spectrum, resulting in minima at 208 and 220 nm, typical for α-helical structure; 10% TFE (□), 20% TFE (◊), 30% TFE (Δ), 40% TFE (x), 50% TFE (○), 60% TFE (+). (B) Turbidimetric changes induced by the addition of the Pex11-Amph peptide to SUV suspensions. Addition of increasing amounts of the Pex11-Amph peptide to neutral vesicles did not significantly alter the turbidity of the solution (Δ—PC liposomes; ϕ—PC/PE liposomes). However, titration of PC/PE/PS/PI/CL SUVs (▪) with the Pex11-Amph peptide resulted in decreased transmittance of the solution. (C) Sequences of mutant peptides Pex11-Amph-Φ and Pex11-Amph-P. Amino-acid substitutions are marked in red. The Pex11-Amph-Φ peptide is predicted to still form an α-helix with a strongly reduced hydrophobic area as depicted by the 3D model of an ideal α-helix. (D) Binding assays of the Pex11-Amph peptide with SUVs of varying phospholipid content (see Supplementary Table III). After incubation with the peptide, the SUVs were collected by centrifugation. The pellet was suspended in buffer (1/10 volume of the initial mixture). Equal volumes of the supernatant (S) and resuspended pellet (P) fractions were loaded per lane. The figure shows a silver stained SDS–polyacrylamide gel. The Pex11-Amph peptide co-sediments with both neutral and negatively charged vesicles, but significantly more peptide binds to the SUVs when negatively charged liposomes resembling the peroxisomal membrane are used (30% peptide bound). Mutant variants of the Pex11-Amph are affected in membrane binding. The Pex11-Amph-Φ peptide co-sediments with liposomes at a reduced level relative to the wild-type peptide. The Pex11-Amph-P mutant peptide does not associate with neutral liposomes and only very small amounts of the mutant peptide co-sedimented with negatively charged vesicles. (E) CD spectrum of the Pex11-Amph-Φ peptide, showing that this mutant peptide is unstructured in phosphate buffer (⧫), similarly to Pex11-Amph. In the presence of increasing amounts of TFE, the CD spectrum of the Pex11-Amph-Φ peptide displays minima at 208 and 220 nm, indicating that some α-helical structure was formed at these conditions; 10% TFE (□), 20% TFE (⋄), 30% TFE (Δ), 40% TFE (x), 50% TFE (○), 60% TFE (+). (F) The CD spectra of the Pex11-Amph-P peptide show that this mutant peptide does not form an α-helix in either phosphate buffer (⧫) or in TFE; 10% TFE (□), 20% TFE (◊), 30% TFE (Δ), 40% TFE (x), 50% TFE (○), 60% TFE (+).