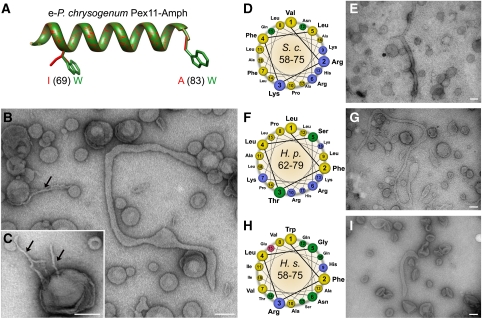
Figure 4.
The membrane remodelling activity of different Pex11-Amph peptides. (A) Superimposition of a 3D model of ideal α-helices of Pex11-Amph (red) and the e-Pex11-Amph mutant (green) with marked mutations. The mutant peptide e-Pex11-Amph contains bulkier tryptophane residues on the hydrophobic interface of the amphipathic helix. (B) Electron micrographs of SUVs of a phospholipid content resembling peroxisomal membranes following incubation with the e-Pex11-Amph peptide. Besides typical tubules observed for Pex11-Amph, low diameter tubules could often be observed (black arrow). (C) The e-Pex11-Amph peptide also induces formation of multiple low diameter tubules developing from single liposomes (black arrows). (D) Helical wheel representation of the region comprising the Pex11-Amph from S. cerevisiae (residues 58–75 of S.c.Pex11) showing the hydrophobic (yellow) and polar, positively charged (blue) interfaces of the helix. (E) Electron micrographs of PC/PE/PS/PI/CL SUVs following incubation with Sc-Pex11-Amph peptide showing extensive tubulation of liposomes. (F) Helical wheel representation of the Pex11-Amph from H. polymorpha (residues 62–79 of Hp-Pex11) revealing the amphipathic properties of the α-helix with a charged polar surface similar to the Pex11-Amph motifs from P. chrysogenum and S. cerevisiae. (G) Incubation of Hp-Pex11-Amph with liposomes with phospholipid content resembling that of the peroxisomal membrane causes efficient tubulation of vesicles. (H) Representation of Pex11-Amph from H. sapiens (residues 58–75 of Hs-Pex11α) in the helical wheel mode shows the amphipathic properties of this motif. In contrast to fungal Pex11-Amph, the corresponding motif in human Pex11α contains a polar interface that is less enriched in basic amino acids. (I) Electron micrograph of PC/PE/PS/PI/CL liposomes after addition of the Hs-Pex11-Amph peptide showing that the amphipathic helix of human Pex11α is active in vesicle tubulation. The observed tubules were usually shorter than for other Pex11-Amph peptides. Scale bars represent 100 nm.