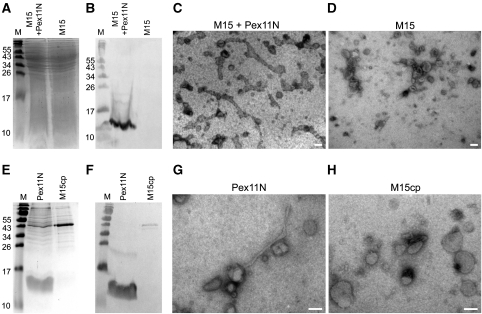
Figure 5.
Membrane remodelling activity of the N-terminal domain of P. chrysogenum Pex11. Coomassie brilliant blue stained SDS–PAA gel (A) and western blot decorated with α-His-tag antibodies (B) of bacterial lysates prepared from E. coli M15 cells producing Pex11N (M15+Pex11N) or the empty host (M15). The western blot shows the presence of a protein band of approximately 12 kDa, which represents Pex11N. Equal amounts of protein were loaded per lane. Electron micrograph of SUVs with a phospholipid content resembling peroxisomal membranes incubated with the bacterial lysate containing Pex11N (M15 + Pex11N; C) showing extensive tubulation of liposomes. Tubulation was not observed when the control lysate prepared from the empty M15 host was used (D). Coomassie brilliant blue stained gel (E) and the corresponding western blot decorated with α-His antibodies (F) showing the protein fraction enriched in Pex11N (Pex11N) and the corresponding control fraction obtained from the empty M15 host (M15cp). Pex11N is detected as a band of approximately 12 kDa both on the Coomassie stained gel and on the western blot. Equal amounts of proteins were loaded per lane. Electron micrographs of peroxisome-like SUVs transformed into tubules after addition of Pex11N protein (G). In the control sample (H; M15cp), liposome tubulation was never observed. The scale bars represent 100 nm.