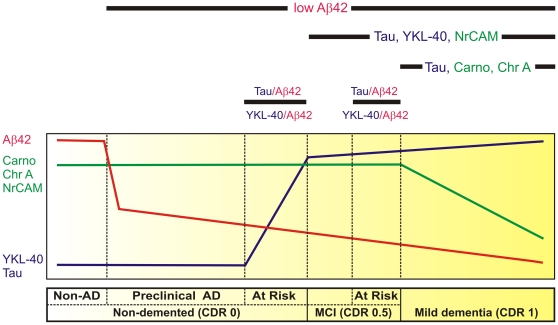
Figure 7. Hypothetical model defines early stages of AD by temporal pattern of CSF protein biomarker levels.
The horizontal bar (below) describes the early clinicopathological progression from cognitive normalcy without AD pathology (‘Non-AD’) to mild dementia in six stages. As depicted by the curves above, Non-AD CSF has high Aβ42 (red line), high chromogranin A (Chr A), carnosinase I (Carno I) and NrCAM (green line), and low YKL-40 and tau (blue line). Reduced CSF Aβ42 correlates with amyloid plaque deposits, the first sign of neuropathologically identifiable AD (‘preclinical AD’) [8]. CSF Aβ42 appears to decrease further as cognition declines from normal (Clinical Dementia Rating [CDR] 0) to very mild cognitive impairment (MCI, CDR 0.5) to mild dementia (CDR 1). When considered as ratios with Aβ42, CSF markers of neuroinflammation (e.g. YKL-40) and neurofibrillary tangle pathology (e.g. tau) appear to increase before and predict the onset of very mild cognitive impairment (MCI, CDR 0.5), defining a CDR 0 group ‘At Risk’ for cognitive decline [9], [15], [137]; YKL-40 and tau also appear to be higher among those who progress rapidly from very mild to mild dementia, defining a CDR 0.5 group ‘At Risk’ for impending cognitive decline [137], [230]. Reductions in synapse-associated (NrCAM, chromogranin A) and neuronal (carnosinase I) proteins, and increases in YKL-40 and tau mirror the progression and anatomical spread of synaptic and neuronal losses, gliosis and tau pathology associated with cognitive decline, and can be used to define CDR 0.5 and CDR 1.