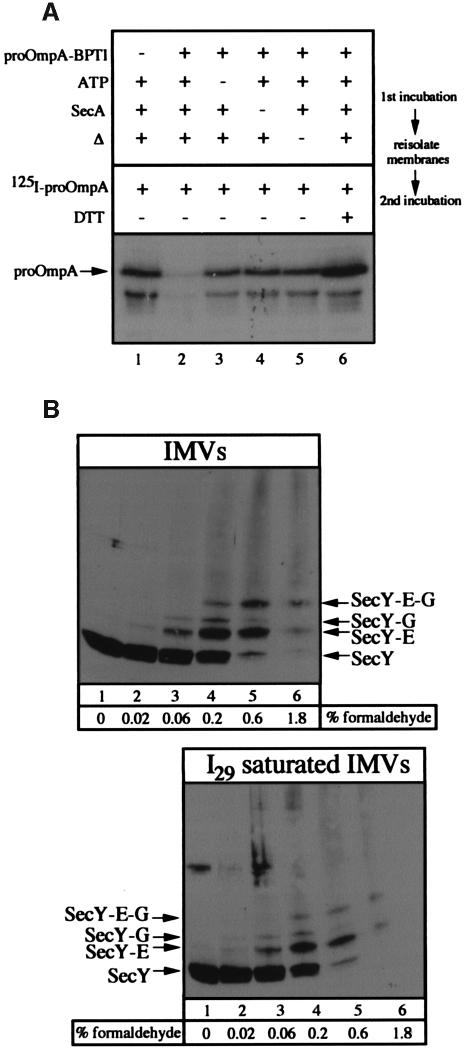
Fig. 2. (A) Saturation of translocase by proOmpA-BPTI. Complete translocation reactions (lanes 2 and 6) or reactions lacking proOmpA-BPTI (lane 1), ATP (lane 3) or SecA (lane 4) were performed at 37°C or on ice (lane 5) as described in Materials and methods. Following a 15 min incubation at 37°C, reactions were layered over 100 µl of TL buffer containing 0.2 M sucrose and centrifuged at 200 000 g for 10 min. Membrane fractions were suspended in TL buffer and supplemented with BSA (200 µg/ml), SecA (50 µg/ml), SecB (66 µg/ml), ATP (2 mM), NADH (5 mM), [125I]proOmpA (50 000 c.p.m.) and DTT (2 mM) (lane 6 only). Following 15 min at 37°C, reactions were transferred to ice and treated with proteinase K (1 mg/ml) for 15 min. Proteins were precipitated with TCA, washed with acetone and analyzed by SDS–PAGE and autoradiography. The position of fully translocated proOmpA is indicated. The stimulation of proOmpA translocation seen in the presence of DTT (lane 6) results from reduction of the disulfide bridge between cysteine residues 290 and 302. (B) Formaldehyde cross-linking of SecYEG. IMVs (160 µg) in 800 µl of HEPES TL buffer or IMVs (160 µg) saturated with I29 translocation intermediate were layered over 200 µl of 0.2 M sucrose in HEPES TL buffer. Membranes were collected by centrifugation (TLA-120.2, 73 000 r.p.m., 10 min) and suspended in 300 µl of HEPES TL buffer. Aliquots of 47.5 µl were removed and treated with formaldehyde to the indicated concentrations for 40 min at 23°C. Following the addition of SDS–PAGE sample buffer, samples were heated for 10 min at 37°C and analyzed by 12% SDS–PAGE and anti-SecY immunoblot analysis. The positions of SecY and SecY cross-linked to SecG and SecE are indicated.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.