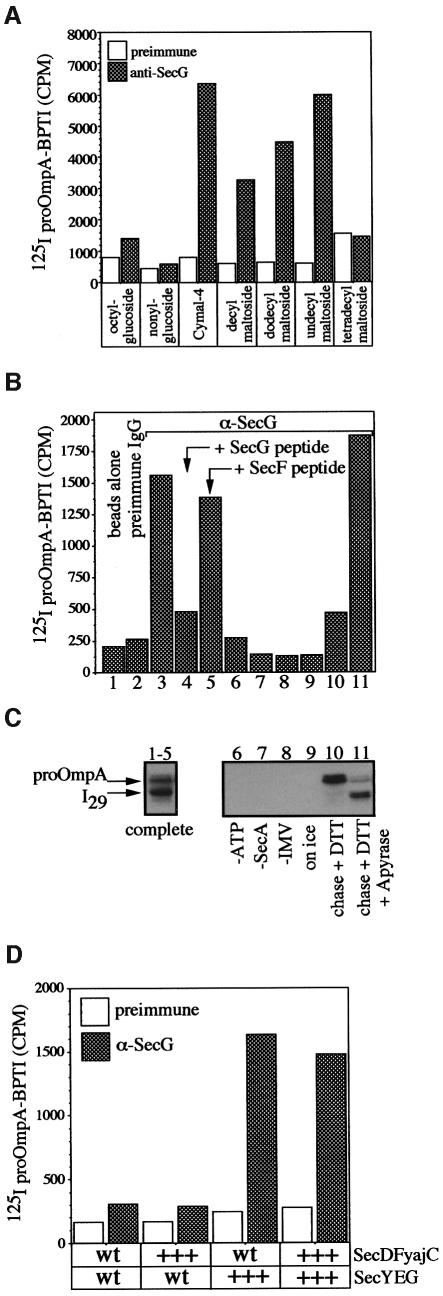
Fig. 3. (A) Solubilization and immunoprecipitation of SecYEG/proOmpA-BPTI. Translocation reactions (100 µl) containing [125I]proOmpA-BPTI (1 × 105 c.p.m.) were performed as described in Materials and methods using IMVs from SecYEG-overexpressing cells. Reactions were solubilized in 50 mM Tris–HCl pH 8.0, 150 mM NaCl, 1.5 mg/ml E.coli phospholipids, 40% glycerol and 1% of the indicated detergents and processed for immunoprecipitation with pre-immune and anti-SecG protein A–Sepharose beads. After a 60 min incubation, beads were washed twice with 1 ml of solubilization buffer and bound proOmpA-BPTI was monitored by scintillation counting. (B and C) Assays of translocation intermediate formation. Complete translocation reactions (lanes 1–5 and 9–11) or reactions lacking either ATP (lane 6), SecA (lane 7) or IMVs (lane 8) were performed at 37°C or on ice (lane 9). After 15 min, potato apyrase (5 U) was added to reaction 11 for 3 min, followed by the addition of DTT (2 mM) and unlabeled proOmpA-BPTI (25 µg/ml) for an additional 10 min at 37°C. Samples were transferred to ice for 5 min, and ATP, SecA or IMV was added to reactions 5, 6 and 7, respectively. An aliquot of each reaction was transferred to ice, treated with proteinase K (10 mg/ml) for 15 min, TCA precipitated and analyzed by SDS–PAGE and autoradiography (C). The remaining portion of each reaction was layered over 0.2 M sucrose in TL buffer and centrifuged (10 min, 200 000 g) to recover membranes. Membrane fractions were solubilized in 500 µl of cymal buffer (50 mM Tris–HCl pH 8.0, 150 mM NaCl, 1.5 mg/ml E.coli phospholipids, 25% glycerol, 1% cymal-4) on ice for 15 min. Samples were centrifuged (100 000 g, 10 min) to remove insoluble material. Soluble extracts were transferred to fresh tubes and incubated with 100 µl of protein A–Sepharose beads alone (reaction 1), beads coupled to pre-immune sera (reaction 2) or affinity-purified anti-SecG IgG (reactions 3–11) for 60 min at 4°C. The beads were washed three times with 1 ml of cymal buffer and proteins were eluted by the addition of 500 µl of 50 mM Tris–HCl pH 8.0, 1% SDS. Bound proOmpA-BPTI was monitored by scintillation counting (B). (D) The effects of membrane composition on the yield of intermediate. Translocation reactions (100 µl) were performed as described above using [125I]proOmpA-BPTI (1 × 105 c.p.m.) mixed with unradiolabeled proOmpA-BPTI (16 µg/ml) and 100 µg/ml of each of the indicated IMVs. Reactions were solubilized in cymal IP buffer and processed for immunoprecipitation with pre-immune and anti-SecG protein A–Sepharose beads. Immunoprecipitated proOmpA-BPTI was monitored by scinitillation counting. wt indicates wild-type levels, while +++ indicates overexpressed levels of the indicated proteins.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.