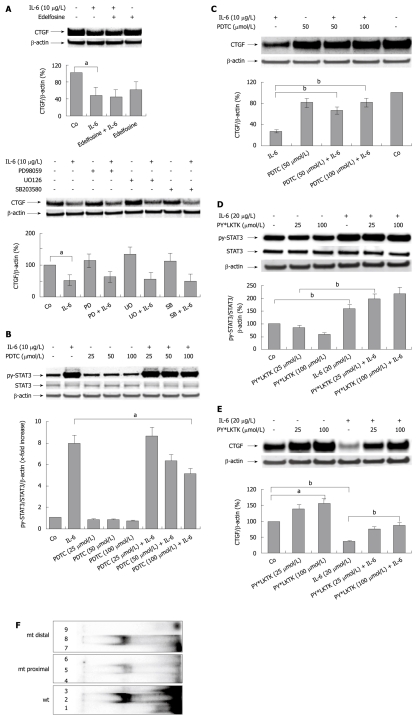
Figure 4.
Interleukin-6 mediates its inhibitory effect on hepatocellular CYR61/CTGF/NOV 2/connective tissue growth factor expression through activation of the STAT3 pathway. A: Western blottings ofCYR61/CTGF/NOV (CCN) 2/connective tissue growth factor (CTGF) of rat hepatocytes (PC) cultured under serum-free conditions with or without addition of the phosphatidylinositol phospholipase C inhibitor edelfosine (10 μmol/L, above) or specific MAP-Kinase inhibitors PD98059 (30 μmol/L), UO126, (10 μmol/L), as well as SB203580, (30 μmol/L) (below) administered to the culture medium 30 min before the addition of rr interleukin (IL)-6 (10 μg/L). Cells were harvested after 24 h. β-actin served as loading control. Blots were quantified relative to β-actin using the Lumi Imager System. A representative blot of 3 independent experiments is shown. aP < 0.005. PD: PD98059; SB: SB203580; UO: UO126; B: Western blottings of phosphorylated and total STAT3 of rat PC cultured under serum-free conditions with or without addition of PDTC at indicated concentrations 2 h prior addition of rrIL-6 (10 μg/L). Cells were harvested after 30 min. β-actin served as loading control. Representative blots of 3 independent cultures are shown. Blots were quantified relative to β-actin using the Lumi Imager System. Quantifications represent the mean ± SD of 3 independent cultures. aP < 0.05 vs IL-6 treated (PDTC untreated) control; C: Western blottings of CCN2/CTGF of rat PC cultured under serum-free conditions with or without addition of PDTC at indicated concentrations 2 h prior addition of rrIL-6 (10 μg/L). Cells were harvested after another 2 h. β-actin served as loading control. A representative blot out of 3 is shown. Blots were quantified as described in (A). bP < 0.0001 vs IL-6 treated (PDTC untreated) control; D: Western blottings of PY-STAT3 and STAT3 of rat PC cultured under serum-free conditions with or without addition of PY*LKTK at indicated concentrations 1 h prior addition of rrIL-6 (20 μg/L). Cells were harvested after another 30 min. β-actin served as loading control. Blots were quantified relative to β-actin using the Lumi Imager System. A representative blot out of 3 is shown. bP < 0.0001; E: Western blottings of CCN2/CTGF of rat PC cultured under serum-free conditions with or without addition of PY*LKTK at indicated concentrations 1 h prior addition of rrIL-6 (20 μg/L). Cells were harvested after another 24 h. β-actin served as loading control. Blots were quantified relative to β-actin using the Lumi Imager System. A representative blot out of 3 is shown. aP < 0.005, bP < 0.0001; F: EMSA using nuclear lysates of PC treated with rrIL-6 (10 μg/L; 30 min) and 32P-labeled double-stranded oligonucleotide probes containing the two proposed wild-type (wt) STAT binding sites as well as the mutated (mt) proximal and distal STAT binding sites in the CTGF promoter. Lane 1: Labeled probe containing both proposed STAT binding sites; lane 2: Nuclear extract and labeled probe containing both proposed STAT binding sites (wt); lane 3: Nuclear extract, labeled probe and 100-fold molar excess of unlabeled probe containing both proposed STAT binding sites (wt); lane 4: Labeled mutated (mt, proximal) probe; lane 5: Nuclear extract and labeled mutated (mt, proximal) probe; lane 6: Nuclear extract, labeled mutated (mt, proximal) probe and 100-fold molar excess of unlabeled mutated (mt, proximal) probe; lane 7: Labeled mutated (mt, distal) probe; lane 8: Nuclear extract and labeled mutated (mt, distal) probe; lane 9: Nuclear extract, labeled mutated (mt, distal) probe and 100-fold molar excess of unlabeled mutated (mt, proximal) probe. The following specific activities were determined using scintillation counting: wt double strand oligonucleotide, 5.23 × 107 cpm/μg DNA; mt proximal oligonucleotide, 3.70 × 107 cpm/μg DNA; mt distal oligonucleotide, 3.73 × 107 cpm/μg DNA. The activities put on the gel were: wt double strand oligonucleotide, 33090 cpm; mt proximal oligonucleotide, 45 844 cpm; mt distal oligonucleotide, 31 556 cpm.