Abstract
AIM: To examine the effects of combined treatment of oxaliplatin and phosphatidylinositol 3’-kinase inhibitor, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) for gastric cancer.
METHODS: Cell viability was evaluated by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. Apoptotic cells were detected by flow cytometric analysis and terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling assay. Western blotting and immuno-precipitation were used to examine protein expression and recruitment, respectively. Nuclear factor κB (NFκB) binding activities were investigated using electrophoretic mobility shift assay. Nude mice were used to investigate tumor growth.
RESULTS: Treatment with combined oxaliplatin and LY294002 resulted in increased cell growth inhibition and cell apoptosis in vitro, and increased tumor growth inhibition and cell death in the tumor mass in vivo. In MKN45 and AGS cells, oxaliplatin treatment promoted both protein kinase B (Akt) and NFκB activation, while pretreatment with LY294002 significantly attenuated oxaliplatin-induced Akt activity and NFκB binding. LY294002 promoted oxaliplatin-induced Fas ligand (FasL) expression, Fas-associated death domain protein recruitment, caspase-8, Bid, and caspase-3 activation, and the short form of cellular caspase-8/FLICE-inhibitory protein (c-FLIPS) inhibition. In vivo, LY294002 inhibited oxaliplatin-induced activation of Akt and NFκB, and increased oxaliplatin-induced expression of FasL, inhibition of c-FLIPS, and activation of caspase-8, Bid, and caspase-3.
CONCLUSION: Combination of oxaliplatin and LY294002 was therapeutically promising for gastric cancer treatment. The enhanced sensitivity of the combined treatment was associated with the activation of the death receptor pathway.
Keywords: Gastric cancer, Oxaliplatin, Phosphatidylinositol 3’-kinase/Akt pathway, Death receptor pathway, Apoptosis, LY294002
INTRODUCTION
Gastric cancer is the second leading cause of cancer death in the world and the top lethal cancer in Asia[1]. The management of gastric cancer is usually a multi-approach involving surgery, chemotherapy, and radiotherapy. Approximately half of gastric cancer patients present with non-operable tumors[2]. As such, besides surgical resection, chemotherapy is the main adjuvant treatment for postoperative and advanced gastric cancer[2]. Combined chemotherapy regimens are currently accepted as the first-line treatment for this disease[3].
Oxaliplatin, a third-generation platinum coordination complex of the 1,2-diaminocyclohexane families, generates covalent adducts between platinum and two adjacent guanines or guanine and adenine in cell DNA, which leads to disruption of DNA replication and transcription[4,5]. Oxaliplatin was shown to be effective in the treatment of advanced gastric cancer when combined with 5-fluorouracil and leucovorin, and has also been used in adjuvant chemotherapy for gastric cancer. Despite the improvement in the efficacy of chemotherapeutic drugs used in the treatment of metastatic gastric cancer, the response rates in the advanced diseases are approximately 47.9% for the most effective drug combinations, and the vast majority of patients relapse, with a median survival of only 11.2 mo[6]. Recently, the combination of chemotherapy and a targeted therapeutic agent was shown to be promising for the treatment of advanced gastric cancer.
Several studies have reported that protein kinase B (Akt) is a key molecule for protecting cells from apoptosis, likely due to phosphorylation and inactivation of a variety of key pro-apoptotic targets. The Akt-mediated survival-signaling pathway is an attractive target for cancer chemotherapy[7-10]. In gastric cancer, over expression and activation of Akt have also been detected, and anomalous expression of Akt induces cell survival[7,11]. In addition, inhibition of Akt activity stimulates apoptosis and enhances the sensitivity of gastric cancer to chemotherapy in a variety of mammalian cells[12-14].
In the present study, we examined the role of 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) in augmenting the anti-cancer effects of oxaliplatin in gastric cancer. We found that LY294002 sensitizes gastric cancer cells to oxaliplatin in both in vitro and in vivo studies. Furthermore, the death receptor pathway was involved in regulating Akt-mediated apoptosis in response to chemotherapy in gastric cancer.
MATERIALS AND METHODS
Cell culture
Human gastric carcinoma cell lines MKN45 and AGS were obtained from the Cell Bank of Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences. All the cell lines were cultured in RPMI 1640 medium (GIBCO, NY, USA) supplemented with heat-inactivated 10% fetal bovine serum (FBS), 10 U/mL penicillin, and 10 μg/mL streptomycin in a humidified atmosphere containing 5% CO2 and 95% air at 37°C.
Antibodies and reagents
Phosphatidylinositol 3’-kinase (PI3K) inhibitor (LY294002) and oxaliplatin were purchased from Alexis Biochemicals (San Diego, CA, USA). The primary antibodies against human Akt1, phosphorylated Akt at Ser473 (phospho-AktSer473), phospho-AktThr308 (Cell Signaling Technology, Beverly, MA, USA), short form of cellular caspase-8/FLICE-inhibitory protein (c-FLIPS), long form of c-FLIP (c-FLIPL), Fas ligand (FasL), Fas, Fas-associated death domain protein (FADD), caspase-8, caspase-3, Bid, nuclear factor κB (NFκB)-p65 and actin (Santa Cruz Biotechnology, Santa Cruz, CA, USA) were used in Western blotting. The primary antibodies against human phospho-AktSer473, NFκB-p65, FasL, active caspase-8, t-Bid, c-FLIPS, and active caspase-3 (Cell Signaling Technology, Beverly, MA, USA) were used in immunohistochemistry.
Cell transfection
FasL siRNA was purchased from Santa Cruz Biotechnology. MKN45 and AGS cells were transiently transfected with FasL siRNA using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturers’ instructions. FasL expression was detected by Western blotting.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay
Cells (4 × 103 cells/well) were plated in 96-well plates in 100 μL of RPMI 1640 without FBS, and incubated for 24 h. Various concentrations (0-4 μmol/L) of the anticancer drugs were added to the culture medium. The viability of cells was evaluated by a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay according to the manufacturers’ specifications (Roche Applied Science, Indianapolis, IN, USA). Briefly, MTT was added at a concentration of 500 mg/L, and cells were incubated for 4 h at 37°C. The absorbance readings of each well were determined using a computer-controlled microtiter plate reader at 570 nmol/L wavelength. The percentage cell survival was defined as the relative absorbance of untreated vs treated cells.
Apoptosis analysis
Cells were treated with various concentrations (0-20 μmol/L) of anticancer drugs and suspended at chosen time points (24 h). Next, 2 × 106 cells were centrifuged and washed twice with ice-cold phosphate-buffered saline. Apoptotic cells were detected by flow cytometry using Annexin V-Fluorescein and propidium iodide (Molecular Probes, Invitrogen, Eugene, OR, USA).
Western blotting and immunoprecipitation
Cells were lysed in ice-cold lysis buffer (25 mmol/L Tris/HCl, pH 7.6, 150 mmol/L NaCl, 5 mmol/L EDTA, 1 mmol/L Na3VO4, 50 mmol/L b-glycerophosphate, 10 mmol/L NaF, 1% Triton X-100, and 0.5 mmol/L phenylmethyl sulfonylfluoride) containing a protease inhibitor cocktail (Roche Diagnostics Ltd., Mannheim, Germany). Protein concentration was determined by Protein Assay (Bio-Rad laboratories, Hercules, California, USA). Western blotting was performed and subjected to the standard protocol. Total cellular proteins (40 μg protein) were separated on SDS-PAGE, and transferred to nitrocellulose membranes (Bio-Rad laboratories). Anti-actin antibody was used to ascertain equal loading of protein. Specific antibodies diluted in TBS-T containing 5% nonfat milk were used to detect indicated proteins. The appropriate horseradish peroxidase (HRP) conjugated secondary antibodies were used at 1:3000 for all antibodies. Positive antibody reactions were detected with the enhanced chemoluminescence system and Hyperfilm X-ray film.
For immunoprecipitation of the Fas death-inducing signaling complex (DISC), cells were lysed and the lysate (300 mg protein/sample) was incubated with 0.4 mg anti-Fas antibody overnight at 4°C. Immunoprecipitates were separated by 10% SDS-PAGE and immunoblotted with anti-FADD.
Electrophoretic mobility shift assay
NFκB binding assays were performed using nuclear extracts and biotin-labeled NFκB oligonucleotides (Panomics, Fremont, CA, USA). Electrophoretic mobility shift assay (EMSA) was performed using an EMSA Gel-Shift Kit. For EMSA, an equal amount of nuclear extracts was incubated for 30 min with an NFκB-specific 32P-labeled oligonucleotide and binding mix as described previously[15]. Samples were electrophoresed at 100 V and 4°C, transferred to Biodyne nylon membranes (Pierce Biotechnology, Rockford, IL, USA), and then cross-linked in an ultraviolet cross-linker (Stratagene Inc., La Jolla, CA, USA). Protein gels were visualized using streptavidin-HRP followed by chemiluminescence detection. The nucleotide sequence of biotin-labeled NFκB was 5'-AGCTATGTGGGTTTTCCCATGAGC-3'.
In vivo assay for tumor growth
MKN45 (5 × 106) was implanted subcutaneously into the flank of nude mice (6 in each group, male BALB/c nu/nu, 4-6 wk of age) (Institute of Materia Medica, CAS, Shanghai, China). When the tumors were 100-150 mm3 in size, oxaliplatin (1.3 mg/kg) and/or LY294002 (25 mg/kg) were injected into the intraperitoneal space every four days. Tumor growth was monitored by measuring tumor volume, which was calculated by the formula: V (mm3) = width2 (mm2) × length (mm)/2. The mice were sacrificed 6 wk later, and tumors were harvested and evaluated with hematoxylin and eosin and terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL) assay. The expression of phospho-AktSer473, p65 subunit of NFκB (NFκB-p65), and several proteins in the death receptor pathway was examined by immunohistochemistry as described previously[16].
TUNEL assay
To detect apoptotic cells in tumor tissue sections, an in situ apoptosis detection kit (Roche Diagnostics) was used. Tumor sections were incubated with proteinase K, rinsed with ddH2O, dewaxed with dimethylbenzene, and rehydrated with gradient ethanol. A 3% H2O2 solution was used to block endogenous peroxidase. After incubation with equilibration buffer and terminal deoxynucleotidyl transferase enzyme, sections were incubated with antidigoxigenin-peroxidase conjugate. Peroxidase activity in each section was shown by diaminobenzidine. Finally, sections were counterstained with hematoxylin. Positive cells were identified and counted (three random fields per slide) under light microscope (Carl Zeiss, Thornwood, NY, USA).
Statistical analysis
All data were expressed as mean ± SD. Comparisons of the difference of mean values were assessed using Student’s two-tailed t test. Differences were considered statistically significant for P < 0.05 and P < 0.01. All means were calculated from at least three independent experiments.
RESULTS
LY294002 increased oxaliplatin-induced cell proliferation inhibition and apoptosis in gastric cancer cells
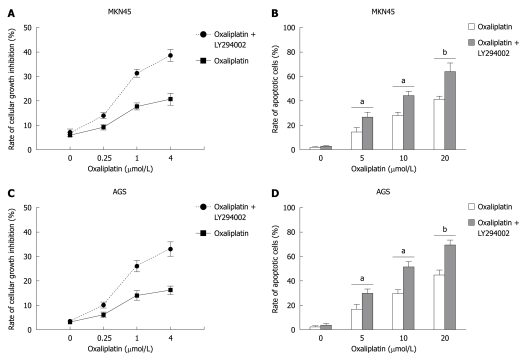
MKN45 and AGS cells were treated with various doses of oxaliplatin (0, 0.25, 1, 4 μmol/L for cell growth inhibition, and 0, 5, 10, 20 μmol/L for cell apoptosis) for 24 h with or without the pretreatment of LY294002 (25 μmol/L). Cell growth inhibition was evaluated by MTT assay. Apoptotic cells were investigated by flow cytometry. LY294002 significantly increased oxaliplatin-induced growth inhibition (In MKN45, oxaliplatin vs oxaliplatin + LY294002: 3.2% ± 0.1% vs 4.1% ± 0.1%, P > 0.05, 6.7% ± 1.1% vs 11.5% ± 1.3%, P < 0.05, 12.5% ± 1.3% vs 29.7% ± 1.7%, P < 0.01, and 13.7% ± 3.1% vs 29.8% ± 3.3%, P < 0.01; in AGS, 6.6% ± 0.1% vs 7.1% ± 0.2%, P > 0.05, 8.4% ± 1.4% vs 14.3% ± 1.2%, P < 0.05, 16.5% ± 2.5% vs 41.1% ± 3.8%, P < 0.01, and 18.4% ± 2.1% vs 35.3% ± 4.3%, P < 0.01) and apoptosis (in MNK45, oxaliplatin vs oxaliplatin + LY294002: 1.7% ± 0.1% vs 2.6% ± 0.3%, P > 0.05, 14.3% ± 3.4% vs 26.3% ± 4.3%, P < 0.05, 28.0% ± 4.7% vs 44.2% ± 5.12%, P < 0.01, and 41.4% ± 4.7% vs 63.1% ± 9.3%, P < 0.01; in AGS, 3.2% ± 0.1% vs 4.1% ± 1.2%, P > 0.05, 13.4% ± 3.8% vs 22.7% ± 3.5%, P < 0.05, 26.6% ± 4.1% vs 42.5% ± 4.8%, P < 0.01, and 40.9% ± 5.9% vs 69.8% ± 6.5%, P < 0.01) (Figure 1).
Figure 1.
LY294002 increased oxaliplatin-induced cell proliferation and apoptosis in gastric cancer cells. MKN45 and AGS cells were treated with various doses of oxaliplatin (0-20 μmol/L) for 24 h with or without LY294002 pretreatment (25 μmol/L). A, C: Cell growth inhibitory rates were evaluated by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay; B, D: Apoptosis of cells was investigated by flow cytometry. LY294002 significantly increased oxaliplatin-induced growth inhibition and apoptosis. aP < 0.05, bP < 0.01 vs oxaliplatin alone.
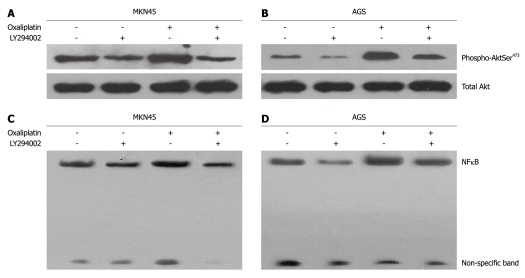
LY294002 inhibited basal and oxaliplatin-induced phosphorylation of Akt and NFκB/DNA binding activities
MKN45 and AGS cells were treated with oxaliplatin (20 μmol/L) and LY294002 (25 μmol/L) used singly or in combination for 24 h. For combined treatment, pretreatment of LY294002 was followed by oxaliplatin. Oxaliplatin induced an increase in the phosphorylation of Akt (Ser473) in MK45 and AGS cells. LY294002 significantly reduced oxaliplatin-induced phosphorylation of Akt (Ser473) (Figure 2A and B). Oxaliplatin and LY294002 did not modulate the phosphorylation of Akt at Thr308 (data not shown). NFκB activity in MKN45 and AGS cells was examined using EMSA. Oxaliplatin stimulated NFκB/DNA binding activity in MKN45 and AGS cells (Figure 2C and D). When oxaliplatin was combined with LY294002, NFκB/DNA binding activity was decreased.
Figure 2.
LY294002 inhibited basal and oxaliplatin-induced phosphorylation of Akt and nuclear factor κB/DNA binding activities. MKN45 and AGS cells were incubated with oxaliplatin (20 μmol/L) or LY294002 (25 μmol/L) used singly or in combination for 24 h. A, B: Oxaliplatin enhanced the phosphorylation of Akt (Ser473, but not Thr308), while LY294002 inhibited the induction of Akt activity in MKN45 and AGS cells; C, D: Oxaliplatin increased nuclear factor κB (NFκB)/DNA binding activity, while LY294002 inhibited the induction of NFκB/DNA binding activity.
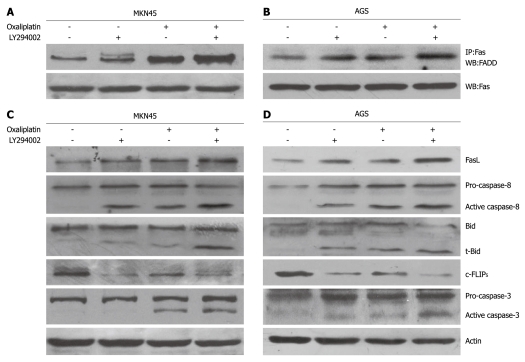
Effects of oxaliplatin, LY294002, or combination in recruitment of FADD, expression of FasL and c-FLIPS, and activation of caspase-8, Bid and caspase-3
Several molecules of the death receptor pathway were investigated using Western blotting. In MKN45 and AGS cells, oxaliplatin increased FasL expression, recruited FADD, and activated caspase-8, caspase-3, and Bid cleavage (t-Bid formation) (Figure 3). LY294002 significantly promoted the oxaliplatin-induced changes. Oxaliplatin reduced the c-FLIPS, while LY294002 enhanced this effect of oxaliplatin. Oxaliplatin and LY294002 did not modulate the expression of the c-FLIPL (data not shown).
Figure 3.
Effects of oxaliplatin, LY294002, or combination on recruitment of Fas-associated death domain protein, expression of Fas ligand and short form of cellular caspase-8/FLICE-inhibitory protein, and activation of caspase-8, Bid, and caspase-3. A-D: In MKN45 and AGS cells, oxaliplatin led to increased Fas ligand (FasL) expression, Fas-associated death domain protein (FADD) recruitment, caspase-8 and caspase-3 activation, and Bid cleavage (t-Bid formation). LY294002 significantly promoted these oxaliplatin-induced changes. Oxaliplatin reduced short form of cellular caspase-8/FLICE-inhibitory protein (c-FLIPS) expression, while LY294002 enhanced this effect of oxaliplatin. Oxaliplatin and LY294002 did not modulate long form of cellular caspase-8/FLICE-inhibitory protein expression (data not shown). IP: Immunoprecipitation; WB: Western blotting.
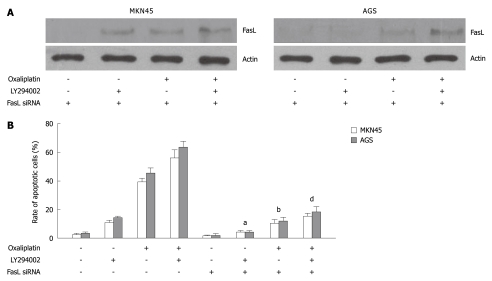
FasL siRNA attenuated oxaliplatin-, LY294002-, or combination-induced cell apoptosis
To further investigate whether LY294002 promoted oxaliplatin-induced apoptosis through the death receptor pathway, MKN45 and AGS cells transfected with FasL siRNA were treated with oxaliplatin, LY294002, or a combination of both. FasL expression was inhibited by FasL siRNA in MKN45 and AGS cells (Figure 4A). FasL silencing decreased LY294002- (in MKN45, LY294002 vs LY294002 + FasL siRNA: 10.5% ± 1.3% vs 4.1% ± 0.6%, P < 0.05; in AGS, 14.6% ± 0.7 vs 4.0% ± 0.7%, P < 0.05), oxaliplatin- (in MKN45, oxaliplatin vs oxaliplatin + FasL siRNA: 39.4% ± 3.6% vs 10.7 % ± 3.9%, P < 0.01; in AGS, 45.1% ± 4.1% vs 11.8% ± 2.8%, P < 0.01), or combination- (in MKN45, combination vs combination + FasL siRNA: 55.7% ± 7.6% vs 15.4% ± 2.4%, P < 0.01; in AGS, 63.4% ± 5.8% vs 18.6% ± 4.5%, P < 0.01) induced cell apoptosis (Figure 4B).
Figure 4.
Fas ligand siRNA attenuated oxaliplatin-, LY294002-, or combination-induced cell apoptosis. A: Fas ligand (FasL) expression was inhibited by FasL siRNA in MKN45 and AGS cells; B: FasL silencing decreased oxaliplatin-, LY294002-, or combination-induced cell apoptosis. aP < 0.05 vs LY294002 treatment; bP < 0.01 vs oxaliplatin treatment; dP < 0.01 vs combination of oxaliplatin and LY294002.
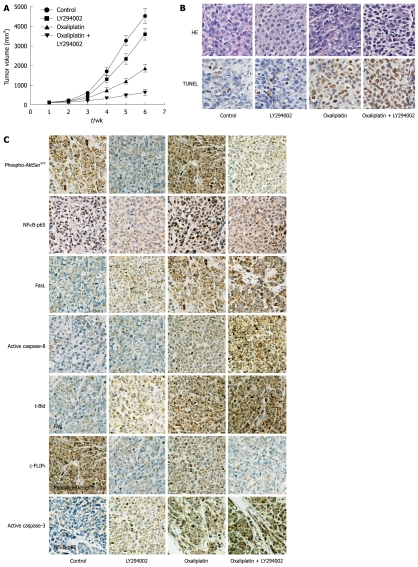
Effects of oxaliplatin, LY294002, or combination on tumor growth and apoptosis in vivo
Four experimental groups were examined: (1) control group; (2) LY294002 group; (3) oxaliplatin group; and (4) combined oxaliplatin and LY294002 therapy group. Tumor growth curves were plotted to compare differences in anti-tumor efficiency in the course of the experiments (Figure 5A). TUNEL assay was performed to detect apoptotic cells in tumor tissue sections (Figure 5B). At the end of 6 wk, tumor volume in combined oxaliplatin and LY294002 therapy group was greatly reduced compared with oxaliplatin group (763 ± 155 mm3 vs 1789 ± 233 mm3, P < 0.01). Oxaliplatin combined with LY294002 significantly enhanced cell death in the tumor mass via apoptosis when compared with oxaliplatin treatment alone.
Figure 5.
Effects of oxaliplatin, LY294002, or combination on in vivo tumor growth and apoptosis. A: Tumor volumes of nude mice in each group are presented. Each time point represents the mean tumor volume for each group; B: Detection of apoptotic cells in tumor tissue was performed by transferase-mediated dUTP nick end labeling (TUNEL) assay; C: The expression of phospho-AktSer473, nuclear factor κB (NFκB)-p65, Fas ligand (FasL), short form of cellular caspase-8/FLICE-inhibitory protein (c-FLIPS), Bid, caspase-8, and caspase-3 was investigated by immunohistochemical analysis.
Immunohistochemical analysis was performed to evaluate the expression of death receptor pathway molecules (Figure 5C). LY294002 inhibited oxaliplatin-induced activation of Akt and NFκB, and increased oxaliplatin-induced expression of FasL, inhibition of c-FLIPS, and activation of caspase-8, Bid and caspase-3.
DISCUSSION
Oxaliplatin is a diaminocyclohexane platinum anti-cancer agent. Although oxaliplatin produces DNA crosslinking similar to those of cisplatin[17], cisplatin-resistant cells generally remain sensitive to oxaliplatin[18]. Furthermore, oxaliplatin induces fewer complications compared with other platinum derivates such as cisplatin and carboplatin that induce nephrotoxicity[19] and myelosuppression[20], respectively. Recently, oxaliplatin was shown to be effective in the treatment of advanced gastric cancer when combined with 5-fluorouracil and leucovorin, and has also been used in adjuvant chemotherapy for gastric cancer. However, despite the improvement in the efficacy of chemotherapeutic drugs used in the treatment of metastatic gastric cancer, the response rate and relative 5-year survival rate in the advanced disease remain low[21].
The PI3K/Akt signaling pathway plays a critical role in cell cycling, cell growth, protein translation, and suppression of apoptosis by Akt-mediated phosphorylation[22-24], and also promotes tumor growth, survival, and aggressiveness[25,26]. In gastric cancer, several studies have reported that the majority of patients exhibit increased expression and activation of Akt[11,27]. Over expression of phosphorylated Akt was associated with poor overall survival, disease-free survival, and high tumor recurrence in gastric cancer patients[28]. In gastric carcinoma cell lines, phosphorylation of Akt is required for cell growth and survival[28]. Thus, blocking the constitutively active PI3K/Akt signaling pathway may provide a novel strategy for targeted cancer therapy.
In this study, the specific PI3K inhibitor LY294002 promoted oxaliplatin-induced growth inhibition and cell apoptosis in MKN45 and AGS cells, suggesting that LY294002 enhanced the chemotherapeutic sensitivity to oxaliplatin in gastric cancer cells. Previous in vitro and in vivo studies demonstrated that activation of the PI3K pathway was associated with the therapeutic efficacy of several chemotherapeutic agents including 5-FU, paclitaxel, cisplatin, irinotecan, and doxorubicin[29-32], while activation of the PI3K pathway induced chemoresistance in cancer cells. To explore the possible mechanisms of LY294002 in sensitizing gastric cancer cells to oxaliplatin, we examined the phosphorylation levels of Akt in oxaliplatin treated MKN45 and AGS cells. We found increased expression of phosphorylated Akt at Ser473 after treatment with oxaliplatin in MKN45 and AGS cells, which is in agreement with a previous study in cholangiocarcinoma cells[33]. LY294002 blocked basal and oxaliplatin-induced phosphorylation of Akt, and resulted in an increased apoptotic rate compared with oxaliplatin alone, suggesting that Akt phosphorylation might regulate oxaliplatin resistance in gastric cancer cells. The significant increase in oxaliplatin-induced cytotoxicity in gastric cancer pretreated with LY294002 indicates that the resistance of gastric cancer cells to chemotherapeutic agents can be modulated.
NFκB plays an important role in suppression of apoptosis. Akt phosphorylates IκB (NFκB inhibitor) kinases, leading to degradation of IκB, as well as NFκB activation[34]. Although many studies strongly support the anti-apoptotic role of NFκB, there are some evidences that NFκB can induce apoptosis[35-37]. In the present study, oxaliplatin enhanced NFκB/DNA binding activity, while LY294002 blocked anticancer drug-induced activation of NFκB. These data indicate that activation of Akt/NFκB in gastric cancer cells may be a key mechanism in inhibiting oxaliplatin-induced apoptosis. It is possible that additional components of the PI3K/Akt pathway may be involved in the chemoresistance of gastric cancer cells.
To further define the role of LY294002 in the regulation of oxaliplatin-induced apoptosis, we examined expression of molecular markers of the death receptor-signaling pathway. LY294002 dramatically increased oxaliplatin-induced FasL expression, FADD redistribution into membrane lipid rafts, caspase-8 and caspase-3 activation, and Bid cleavage in MKN45 and AGS cells. Next, we down-regulated FasL using FasL siRNA in LY294002-, oxaliplatin-, or combination-treated MKN45 and AGS cells. Oxaliplatin, LY294002, or combination treatment-induced apoptosis was attenuated by FasL silencing, suggesting that the death receptor pathway might be involved in the cell apoptosis induced by oxaliplatin or LY294002 in gastric cancer cells. However, the precise mechanism whereby oxaliplatin or LY294002 induces FasL expression remains unknown.
Apoptosis mediated by Fas is regulated by c-FLIP expression[38]. There are two isoforms of c-FLIP: the full-length c-FLIPL and c-FLIPS[39,40]. c-FLIPS is considered solely anti-apoptotic and confers resistance to receptor-mediated apoptosis by blocking proteolytic activation of caspase-8 at the Fas DISC, while c-FLIPL exhibits dual roles[41,42]. Additionally, c-FLIPS and c-FLIPL are differently regulated[43-45]. The PI3K pathway is an important regulator of c-FLIPS, but not c-FLIPL, expression in human gastric cancer cells[45]. In this study, oxaliplatin-induced apoptotic death was accompanied by suppression of c-FLIPS in MKN45 and AGS cells. Compared with oxaliplatin alone, combination of oxaliplatin and LY294002 produced enhanced down-regulation of c-FLIPS. c-FLIPL expression was not significantly changed by treatment with LY294002 or oxaliplatin. These findings indicate that the anti-apoptotic function of c-FLIPS may be more potent than that of c-FLIPL in oxaliplatin-induced apoptosis, and that Akt is involved in regulation of c-FLIPS in human gastric cancer cells.
We also examined the effects of the combined treatment of oxaliplatin and LY294002 in an in vivo xenograft model. LY29400 significantly increased oxaliplatin-induced tumor growth and cell death in the tumor mass via apoptosis. Moreover, altered expression levels of FasL, Bid, caspase-8, caspase-3, and c-FLIPS were found in the tumor xenograft. These data suggest that combination of oxaliplatin and LY294002 elicited a strong antitumor effect in gastric cancer in vivo, and that the death receptor pathway might mediate the additive cytotoxicity of oxaliplatin and LY294002.
In summary, we present a novel therapeutic approach for treatment of gastric cancer using the combined oxaliplatin and the PI3K/Akt inhibitor LY294002, that may be mediated, at least in part, by modification of the death receptor pathway.
COMMENTS
Background
Gastric cancer remains a leading cause of cancer death worldwide. Besides surgical resection, chemotherapy is important treatment for gastric cancers. Despite the improvement in the efficacy of chemotherapeutic drugs, the response rates and the median survival remain low.
Research frontiers
Traditional cancer therapy predominantly utilizes cytotoxic chemotherapeutic agents. The cytotoxic events are affected mainly through disruption of various aspects of DNA synthesis and repair or disturbance of mitosis, processes which are common to all dividing cells. For this reason, most chemotherapeutic agents are often accompanied with substantial adverse effects. Target-protein-based cancer therapy has become available in clinical practice. Phosphatidylinositol 3’-kinase (PI3K) inhibitors have potential to target specific pathways involved in tumor cell growth.
Innovations and breakthroughs
The PI3K/Akt pathway has been shown to be involved in the chemoresistance of gastric cancer. In both in vitro and in vivo studies, the targeted inhibition of PI3K/Akt results in increased oxaliplatin-induced apoptosis and inhibition of cellular proliferation of gastric cancer. Furthermore, the activation of the death receptor pathway may be an important mechanism by which PI3K/Akt inhibition is involved in oxaliplatin-induced apoptosis.
Applications
By understanding how LY294002 enhances the therapeutic effect of oxaliplatin in gastric cancer cells, this study provides information about the potential therapeutic intervention in patients with gastric adenocarcinoma.
Terminology
Oxaliplatin: A third-generation platinum coordination complex of the 1,2-diaminocyclohexane families, generates covalent adducts between platinum and two adjacent guanines or guanine and adenine in cell DNA. LY294002: 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one, a specific inhibitor of PI3K.
Peer review
This is a well-written report on the synergistic anti-tumor effects of the combined treatment with oxaliplatin and LY294002 in gastric cancer cells. The data and results are straight-forward and clearly support the conclusion that targeting PI3K/Akt results in increased oxaliplatin-induced apoptosis and inhibition of cellular proliferation of gastric cancer.
Acknowledgments
The authors thank Hong Xia (Institute for Gastroenterology and Hepatology, Wuhan University Medical School) for his valuable discussions and suggestions.
Footnotes
Supported by The National Natural Science Foundation of China, No. 30470782
Peer reviewers: Jun-Hyeog Jang, Professor, Chief, Department of Biochemistry, Inha University School of Medicine, Jung-Gu, Incheon 400-712, South Korea; Long-Bin Jeng, MD, Organ Transplantation Center, China Medical University Hospital, 2 Yuh-Der Road, Taichung 40447, Taiwan, China; Tatsuo Kanda, MD, PhD, Division of Digestive and General Surgery, Graduate School of Medical and Dental Sciences, Niigata University, Niigata City 951-8510, Japan
S- Editor Sun H L- Editor Ma JY E- Editor Zheng XM
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Hundahl SA, Phillips JL, Menck HR. The National Cancer Data Base Report on poor survival of U.S. gastric carcinoma patients treated with gastrectomy: Fifth Edition American Joint Committee on Cancer staging, proximal disease, and the "different disease" hypothesis. Cancer. 2000;88:921–932. [PubMed] [Google Scholar]
- 3.Sasako M, Inoue M, Lin JT, Khor C, Yang HK, Ohtsu A. Gastric Cancer Working Group report. Jpn J Clin Oncol. 2010;40 Suppl 1:i28–i37. doi: 10.1093/jjco/hyq124. [DOI] [PubMed] [Google Scholar]
- 4.Boudný V, Vrána O, Gaucheron F, Kleinwachter V, Leng M, Brabec V. Biophysical analysis of DNA modified by 1,2-diaminocyclohexane platinum(II) complexes. Nucleic Acids Res. 1992;20:267–272. doi: 10.1093/nar/20.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kollmannsberger C, Quietzsch D, Haag C, Lingenfelser T, Schroeder M, Hartmann JT, Baronius W, Hempel V, Clemens M, Kanz L, et al. A phase II study of paclitaxel, weekly, 24-hour continous infusion 5-fluorouracil, folinic acid and cisplatin in patients with advanced gastric cancer. Br J Cancer. 2000;83:458–462. doi: 10.1054/bjoc.2000.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cunningham D, Starling N, Rao S, Iveson T, Nicolson M, Coxon F, Middleton G, Daniel F, Oates J, Norman AR. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358:36–46. doi: 10.1056/NEJMoa073149. [DOI] [PubMed] [Google Scholar]
- 7.Oki E, Baba H, Tokunaga E, Nakamura T, Ueda N, Futatsugi M, Mashino K, Yamamoto M, Ikebe M, Kakeji Y, et al. Akt phosphorylation associates with LOH of PTEN and leads to chemoresistance for gastric cancer. Int J Cancer. 2005;117:376–380. doi: 10.1002/ijc.21170. [DOI] [PubMed] [Google Scholar]
- 8.Yang X, Fraser M, Moll UM, Basak A, Tsang BK. Akt-mediated cisplatin resistance in ovarian cancer: modulation of p53 action on caspase-dependent mitochondrial death pathway. Cancer Res. 2006;66:3126–3136. doi: 10.1158/0008-5472.CAN-05-0425. [DOI] [PubMed] [Google Scholar]
- 9.Burk DT, Bender DJ. Use and perceived effectiveness of student support services in a first-year dental student population. J Dent Educ. 2005;69:1148–1160. [PubMed] [Google Scholar]
- 10.Stassi G, Garofalo M, Zerilli M, Ricci-Vitiani L, Zanca C, Todaro M, Aragona F, Limite G, Petrella G, Condorelli G. PED mediates AKT-dependent chemoresistance in human breast cancer cells. Cancer Res. 2005;65:6668–6675. doi: 10.1158/0008-5472.CAN-04-4009. [DOI] [PubMed] [Google Scholar]
- 11.Ang KL, Shi DL, Keong WW, Epstein RJ. Upregulated Akt signaling adjacent to gastric cancers: implications for screening and chemoprevention. Cancer Lett. 2005;225:53–59. doi: 10.1016/j.canlet.2004.11.021. [DOI] [PubMed] [Google Scholar]
- 12.Vega F, Medeiros LJ, Leventaki V, Atwell C, Cho-Vega JH, Tian L, Claret FX, Rassidakis GZ. Activation of mammalian target of rapamycin signaling pathway contributes to tumor cell survival in anaplastic lymphoma kinase-positive anaplastic large cell lymphoma. Cancer Res. 2006;66:6589–6597. doi: 10.1158/0008-5472.CAN-05-3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia GE, Nicole A, Bhaskaran S, Gupta A, Kyprianou N, Kumar AP. Akt-and CREB-mediated prostate cancer cell proliferation inhibition by Nexrutine, a Phellodendron amurense extract. Neoplasia. 2006;8:523–533. doi: 10.1593/neo.05745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang HM, Rao JN, Guo X, Liu L, Zou T, Turner DJ, Wang JY. Akt kinase activation blocks apoptosis in intestinal epithelial cells by inhibiting caspase-3 after polyamine depletion. J Biol Chem. 2004;279:22539–22547. doi: 10.1074/jbc.M314337200. [DOI] [PubMed] [Google Scholar]
- 15.Vanden Berghe W, De Bosscher K, Boone E, Plaisance S, Haegeman G. The nuclear factor-kappaB engages CBP/p300 and histone acetyltransferase activity for transcriptional activation of the interleukin-6 gene promoter. J Biol Chem. 1999;274:32091–32098. doi: 10.1074/jbc.274.45.32091. [DOI] [PubMed] [Google Scholar]
- 16.Chen Y, Wang Z, Chang P, Xiang L, Pan F, Li J, Jiang J, Zou L, Yang L, Bian Z, et al. The effect of focal adhesion kinase gene silencing on 5-fluorouracil chemosensitivity involves an Akt/NF-kappaB signaling pathway in colorectal carcinomas. Int J Cancer. 2010;127:195–206. doi: 10.1002/ijc.25025. [DOI] [PubMed] [Google Scholar]
- 17.Almeida GM, Duarte TL, Steward WP, Jones GD. Detection of oxaliplatin-induced DNA crosslinks in vitro and in cancer patients using the alkaline comet assay. DNA Repair (Amst) 2006;5:219–225. doi: 10.1016/j.dnarep.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 18.Scheeff ED, Briggs JM, Howell SB. Molecular modeling of the intrastrand guanine-guanine DNA adducts produced by cisplatin and oxaliplatin. Mol Pharmacol. 1999;56:633–643. [PubMed] [Google Scholar]
- 19.Legallicier B, Leclere C, Monteil C, Elkaz V, Morin JP, Fillastre JP. The cellular toxicity of two antitumoural agents derived from platinum, cisplatinum versus oxaliplatinum, on cultures of tubular proximal cells. Drugs Exp Clin Res. 1996;22:41–50. [PubMed] [Google Scholar]
- 20.Christian MC. The current status of new platinum analogs. Semin Oncol. 1992;19:720–733. [PubMed] [Google Scholar]
- 21.Wagner AD, Grothe W, Haerting J, Kleber G, Grothey A, Fleig WE. Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J Clin Oncol. 2006;24:2903–2909. doi: 10.1200/JCO.2005.05.0245. [DOI] [PubMed] [Google Scholar]
- 22.Hartmann W, Küchler J, Koch A, Friedrichs N, Waha A, Endl E, Czerwitzki J, Metzger D, Steiner S, Wurst P, et al. Activation of phosphatidylinositol-3'-kinase/AKT signaling is essential in hepatoblastoma survival. Clin Cancer Res. 2009;15:4538–4545. doi: 10.1158/1078-0432.CCR-08-2878. [DOI] [PubMed] [Google Scholar]
- 23.Gao N, Flynn DC, Zhang Z, Zhong XS, Walker V, Liu KJ, Shi X, Jiang BH. G1 cell cycle progression and the expression of G1 cyclins are regulated by PI3K/AKT/mTOR/p70S6K1 signaling in human ovarian cancer cells. Am J Physiol Cell Physiol. 2004;287:C281–C291. doi: 10.1152/ajpcell.00422.2003. [DOI] [PubMed] [Google Scholar]
- 24.Krystal GW, Sulanke G, Litz J. Inhibition of phosphatidylinositol 3-kinase-Akt signaling blocks growth, promotes apoptosis, and enhances sensitivity of small cell lung cancer cells to chemotherapy. Mol Cancer Ther. 2002;1:913–922. [PubMed] [Google Scholar]
- 25.Su JD, Mayo LD, Donner DB, Durden DL. PTEN and phosphatidylinositol 3'-kinase inhibitors up-regulate p53 and block tumor-induced angiogenesis: evidence for an effect on the tumor and endothelial compartment. Cancer Res. 2003;63:3585–3592. [PubMed] [Google Scholar]
- 26.Polo ML, Arnoni MV, Riggio M, Wargon V, Lanari C, Novaro V. Responsiveness to PI3K and MEK inhibitors in breast cancer. Use of a 3D culture system to study pathways related to hormone independence in mice. PLoS One. 2010;5:e10786. doi: 10.1371/journal.pone.0010786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 28.Kobayashi I, Semba S, Matsuda Y, Kuroda Y, Yokozaki H. Significance of Akt phosphorylation on tumor growth and vascular endothelial growth factor expression in human gastric carcinoma. Pathobiology. 2006;73:8–17. doi: 10.1159/000093087. [DOI] [PubMed] [Google Scholar]
- 29.Shin JY, Kim JO, Lee SK, Chae HS, Kang JH. LY294002 may overcome 5-FU resistance via down-regulation of activated p-AKT in Epstein-Barr virus-positive gastric cancer cells. BMC Cancer. 2010;10:425. doi: 10.1186/1471-2407-10-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alexia C, Bras M, Fallot G, Vadrot N, Daniel F, Lasfer M, Tamouza H, Groyer A. Pleiotropic effects of PI-3' kinase/Akt signaling in human hepatoma cell proliferation and drug-induced apoptosis. Ann N Y Acad Sci. 2006;1090:1–17. doi: 10.1196/annals.1378.001. [DOI] [PubMed] [Google Scholar]
- 31.Pencreach E, Guérin E, Nicolet C, Lelong-Rebel I, Voegeli AC, Oudet P, Larsen AK, Gaub MP, Guenot D. Marked activity of irinotecan and rapamycin combination toward colon cancer cells in vivo and in vitro is mediated through cooperative modulation of the mammalian target of rapamycin/hypoxia-inducible factor-1alpha axis. Clin Cancer Res. 2009;15:1297–1307. doi: 10.1158/1078-0432.CCR-08-0889. [DOI] [PubMed] [Google Scholar]
- 32.Karam AK, Santiskulvong C, Fekete M, Zabih S, Eng C, Dorigo O. Cisplatin and PI3kinase inhibition decrease invasion and migration of human ovarian carcinoma cells and regulate matrix-metalloproteinase expression. Cytoskeleton (Hoboken) 2010;67:535–544. doi: 10.1002/cm.20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leelawat K, Narong S, Udomchaiprasertkul W, Leelawat S, Tungpradubkul S. Inhibition of PI3K increases oxaliplatin sensitivity in cholangiocarcinoma cells. Cancer Cell Int. 2009;9:3. doi: 10.1186/1475-2867-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu HG, Ai YW, Yu LL, Zhou XD, Liu J, Li JH, Xu XM, Liu S, Chen J, Liu F, et al. Phosphoinositide 3-kinase/Akt pathway plays an important role in chemoresistance of gastric cancer cells against etoposide and doxorubicin induced cell death. Int J Cancer. 2008;122:433–443. doi: 10.1002/ijc.23049. [DOI] [PubMed] [Google Scholar]
- 35.Ravi R, Bedi GC, Engstrom LW, Zeng Q, Mookerjee B, Gélinas C, Fuchs EJ, Bedi A. Regulation of death receptor expression and TRAIL/Apo2L-induced apoptosis by NF-kappaB. Nat Cell Biol. 2001;3:409–416. doi: 10.1038/35070096. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y, Cui H, Schroering A, Ding JL, Lane WS, McGill G, Fisher DE, Ding HF. NF-kappa B2 p100 is a pro-apoptotic protein with anti-oncogenic function. Nat Cell Biol. 2002;4:888–893. doi: 10.1038/ncb872. [DOI] [PubMed] [Google Scholar]
- 37.Kimura M, Haisa M, Uetsuka H, Takaoka M, Ohkawa T, Kawashima R, Yamatsuji T, Gunduz M, Kaneda Y, Tanaka N, et al. TNF combined with IFN-alpha accelerates NF-kappaB-mediated apoptosis through enhancement of Fas expression in colon cancer cells. Cell Death Differ. 2003;10:718–728. doi: 10.1038/sj.cdd.4401219. [DOI] [PubMed] [Google Scholar]
- 38.Thorburn A. Death receptor-induced cell killing. Cell Signal. 2004;16:139–144. doi: 10.1016/j.cellsig.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 39.Peter ME, Krammer PH. The CD95(APO-1/Fas) DISC and beyond. Cell Death Differ. 2003;10:26–35. doi: 10.1038/sj.cdd.4401186. [DOI] [PubMed] [Google Scholar]
- 40.Thome M, Tschopp J. Regulation of lymphocyte proliferation and death by FLIP. Nat Rev Immunol. 2001;1:50–58. doi: 10.1038/35095508. [DOI] [PubMed] [Google Scholar]
- 41.Kim JY, Kim EH, Park SS, Lim JH, Kwon TK, Choi KS. Quercetin sensitizes human hepatoma cells to TRAIL-induced apoptosis via Sp1-mediated DR5 up-regulation and proteasome-mediated c-FLIPS down-regulation. J Cell Biochem. 2008;105:1386–1398. doi: 10.1002/jcb.21958. [DOI] [PubMed] [Google Scholar]
- 42.Kim DJ, Park C, Oh B, Kim YY. Association of TRAF2 with the short form of cellular FLICE-like inhibitory protein prevents TNFR1-mediated apoptosis. J Mol Signal. 2008;3:2. doi: 10.1186/1750-2187-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bin L, Li X, Xu LG, Shu HB. The short splice form of Casper/c-FLIP is a major cellular inhibitor of TRAIL-induced apoptosis. FEBS Lett. 2002;510:37–40. doi: 10.1016/s0014-5793(01)03222-7. [DOI] [PubMed] [Google Scholar]
- 44.Park SJ, Kim YY, Ju JW, Han BG, Park SI, Park BJ. Alternative splicing variants of c-FLIP transduce the differential signal through the Raf or TRAF2 in TNF-induced cell proliferation. Biochem Biophys Res Commun. 2001;289:1205–1210. doi: 10.1006/bbrc.2001.6086. [DOI] [PubMed] [Google Scholar]
- 45.Nam SY, Jung GA, Hur GC, Chung HY, Kim WH, Seol DW, Lee BL. Upregulation of FLIP(S) by Akt, a possible inhibition mechanism of TRAIL-induced apoptosis in human gastric cancers. Cancer Sci. 2003;94:1066–1073. doi: 10.1111/j.1349-7006.2003.tb01402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]