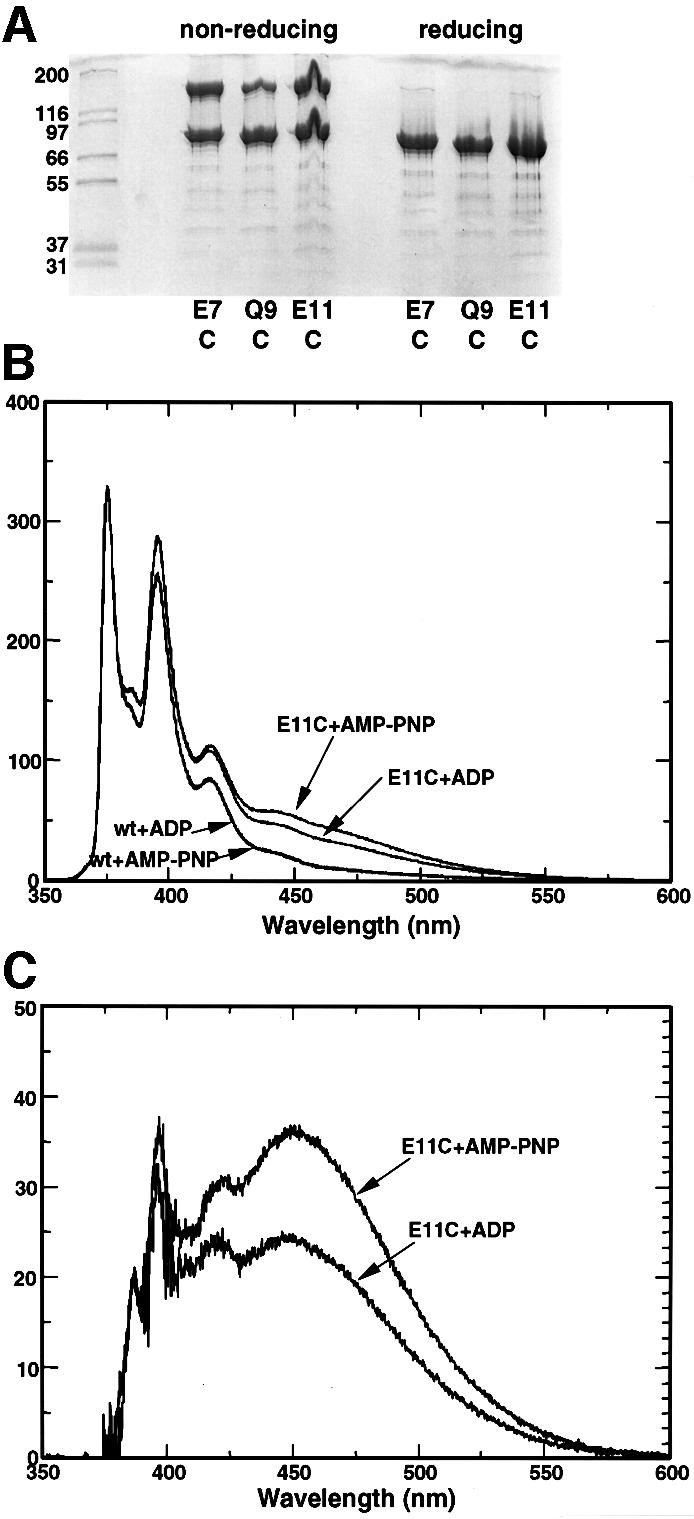
Fig. 4. N-terminal interactions in single-cysteine mutants. (A) Polyacrylamide gel of purified single-cysteine mutants E7C, Q9C and E11C without added nucleotide, under non-reducing conditions and in the presence of the non-thiol reducing agent TCEP. In all three mutants, intra-dimer disulfide bonds are formed in the absence of reducing agent. (B) Excimer formation in pyrene-modified single-cysteine mutants. E11C mutant and wild-type Hsp90s were treated with pyrene (see Materials and methods), and their fluorescence emission spectra recorded. Only the cysteine mutants displayed a significant excimer signal in the region 450–500 nm, and this was substantially enhanced in the presence of AMP-PNP compared with ADP. (C) Specific excimer fluorescence signals for the E11C mutant, corrected for non-specific signal due to adsorption of the hydrophobic pyrene onto the protein surface by subtraction of the corresponding emission spectrum for the wild-type Hsp90.
