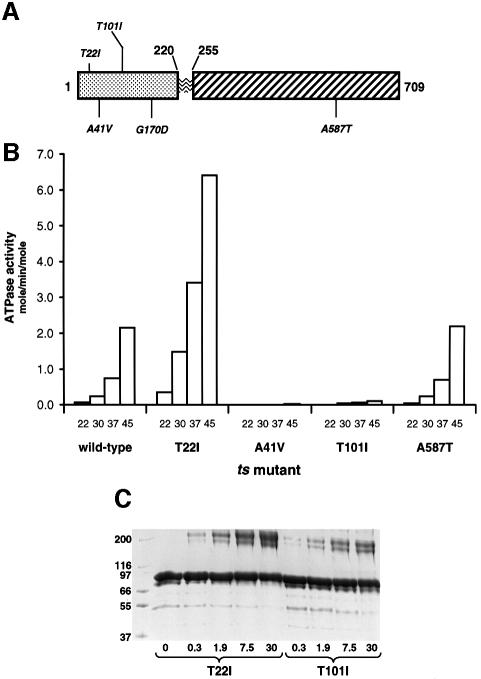
Fig. 6. ATPase activities and dimerization efficiency of ts missense mutants. (A) Schematic of yeast Hsp90 primary structure with positions of missense mutations conferring ts phenotypes indicated. (B) ATPase activities of wild-type Hsp90 and ts mutants at 22, 30, 37 and 45°C. (C) Polyacrylamide gel of Hsp90 ATPase mutants T22I and T101I (6 µM) cross-linked with DMS in the presence of 10 mM AMP-PNP at 37°C for 1 h (see Materials and methods). Numbers below the lanes indicate the molar ratio of cross-linker to protein primary amine content in that reaction. At all linker concentrations the ATPase hyperactive mutant T22I shows a much higher degree of cross-linked dimers than the hypoactive T101I mutant.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.