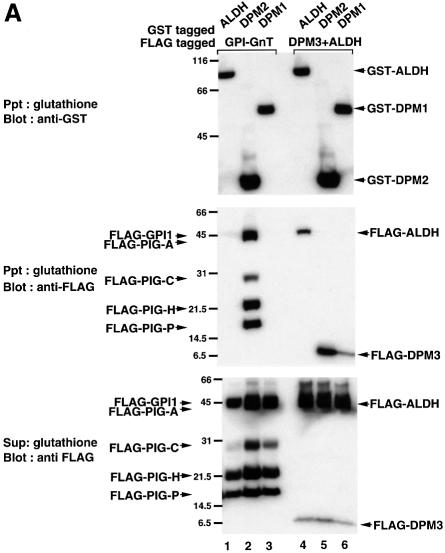
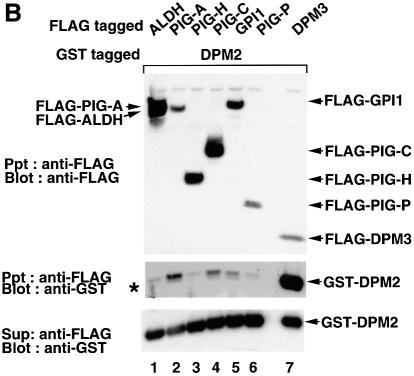
Fig. 5. (A) Interaction of DPM2 with GPI-GnT complexes. GST–ALDH (lanes 1 and 4), –DPM2 (lanes 2 and 5) and –DPM1 (lanes 3 and 6) were co-expressed in JY5 cells with the five FLAG-tagged GPI-GnT components (lanes 1–3), or FLAG-DPM3 plus -ALDH (lanes 4–6). Physical associations between GST- and FLAG-tagged proteins were analyzed by co-precipitation. The lysates in 1% digitonin were precipitated with glutathione beads, and precipitates were analyzed by western blotting against anti-GST (top panel) and anti-FLAG (middle) antibodies. FLAG-tagged proteins remaining in the supernatant after precipitation with glutathione beads were immunoprecipitated and analyzed by western blotting against anti-FLAG antibody (bottom). Positions of molecular size markers are indicated on the left (kDa). (B) Association of DPM2 with components of GPI-GnT. GST–DPM2 was co-expressed in JY5 cells with FLAG-ALDH (lane 1), -PIG-A (lane 2), -PIG-H (lane 3), -PIG-C (lane 4), -GPI1 (lane 5) -PIG-P (lane 6) or -DPM3 (lane 7), and their associations were analyzed by co-precipitation. The lysates were precipitated with anti-FLAG and the immunoprecipitates were analyzed by western blotting against anti-FLAG (top panel) and anti-GST (middle) antibodies. GST–DPM2 remaining in the supernatants after immunoprecipitation was assessed by precipitation with glutathione beads and western blotting (bottom). An asterisk in the middle panel indicates a faint band seen in lane 1 showing a background level of GST–DPM2 present in the precipitates.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.