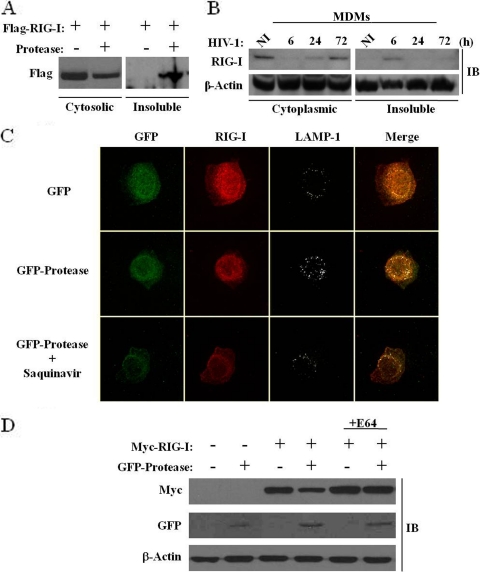
FIG. 8.
HIV-1 protease expression causes a relocalization of the cytoplasmic pool of RIG-I to the lysosomes. (A and B) HEK293 cells were cotransfected with plasmids expressing Flag-RIG-I and GFP-protease (A), and MDMs were infected de novo (or not) with HIV-1 at an MOI of 1 for 6, 24, and 72 h (B). Whole-cell extracts isolated from the cytoplasmic fraction versus the insoluble fraction were run on an SDS-PAGE gel and subsequently subjected to immunoblotting with anti-Flag, anti-RIG-I, or anti-β-actin antibodies, respectively. (C) A549 cells were transfected with plasmids expressing GFP or GFP-protease with or without saquinavir (5 μM). At 15 h posttransfection, cells were, fixed, permeabilized and immunostained for endogenous RIG-I (red) or LAMP-1 (gray). Cells were visualized by confocal microscopy. (D) A lysosomal inhibitor restores RIG-I protein expression. HEK293 cells were cotransfected with Myc-RIG-I or GFP-protease expression plasmids and treated with 10 μM E64 (lysosomal-protease inhibitor cocktail). At 24 h posttransfection, expression levels of RIG-I, protease, and β-actin were analyzed by immunoblotting with antibodies against Myc, GFP, and β-actin, respectively.